SUMMARY
Plasticity in growth and reproductive behavior is found in many vertebrate species, but is common in male teleost fish. Typically, “bourgeois” males are considerably larger and defend breeding territories while “parasitic” variants are small and use opportunistic breeding strategies. The P locus mediates this phenotypic variation in Xipophorus and encodes variant alleles of the melanocortin-4 receptor (MC4R). However, deletion of the MC4R has modest effects on somatic growth and reproduction in mammals, suggesting a fundamental difference in the neuroendocrine function of central melanocortin signaling in teleosts. Here we show in a teleost that the hypothalamic proopiomelanocortin and AgRP neurons are hypophysiotropic, projecting to the pituitary to coordinately regulate multiple pituitary hormones. Indeed, AgRP-mediated suppression of MC4R appears essential for early larval growth. This identifies the mechanism by which the central melanocortin system coordinately regulates growth and reproduction in teleosts, and suggests it is an important anatomical substrate for evolutionary adaptation.
INTRODUCTION
Melanocortin-4 receptor (MC4R) signaling regulates energy homeostasis in vertebrate species from teleosts to humans (Farooqi et al., 2003; Huszar et al., 1997; Song and Cone, 2007; Vaisse et al., 1998; Yeo et al., 1998). The receptor is also known to regulate somatic growth, from modest effects demonstrated in mammals (Farooqi et al., 2003; Huszar et al., 1997), to variant alleles in fish that can double the final length of animals (Lampert et al.). Increased linear growth as a result of disruption of MC4R signaling has been reported in multiple mouse models including AgRP transgenic mice (Graham et al., 1997) and MC4R knockout mice (Huszar et al., 1997). Early studies in humans indicated an increased linear growth rate as early as a few months of age, when children with MC4R haploinsufficiency were compared with control obese children (Farooqi et al., 2003). More recently, MC4R haploinsufficiency in humans has also been demonstrated to be associated with a greater final attained height, relative to MC4R +/+ individuals matched for BMI. In human MC4R haploinsufficiency, there is no measurable increase in Insulin-like Growth Factor I, and hyperinsulinemia has been suggested as a possible mechanism for the increased growth (Martinelli et al., 2011).
The central melanocortin system has also been demonstrated to be highly conserved in fish. The receptors and ligands are expressed in a highly conserved pattern relative to mammals (Forlano and Cone, 2007; Song et al., 2003), and expression of the orexigenic melanocortin antagonist agouti related protein (AgRP) is dramatically upregulated during fasting in goldfish (Cerda-Reverter and Peter, 2003) and zebrafish (Song et al., 2003), also as reported in mammals. Administration of MC4R agonists NDP-MSH or MTII inhibit food intake in goldfish (Cerda-Reverter et al., 2003b) and rainbow trout (Schjolden et al., 2009), while ICV injection of MC4R antagonists, HS024 or SHU9119 stimulates food intake in fed goldfish (Cerda-Reverter et al., 2003b) or rainbow trout (Schjolden et al., 2009). Experimental blockade of the MC4R by ectopic over-expression of AgRP increased body weight, body fat and adult length in zebrafish (Song and Cone, 2007).
Recently, natural mutations affecting melanocortin signaling have also been characterized in fish. In the swordtail fish, X. nigrensis and X. multilineatus, small and large male morphs map to a single locus, P (Kallman and Borkoski, 1978), recently demonstrated to encode the MC4R (Lampert et al.). Large male morphs in this species result from multiple copies of mutant forms of the receptor, at the Y chromosome-encoded P locus, that appear to function in a dominant negative fashion, blocking activity of the wild-type receptor. Remarkably, these single gene mutations also lead to altered onset of puberty and divergent reproductive strategies in the small and large size morphs. Because of the ease of genetic manipulation in the larval zebrafish, we sought to use this model system to better understand the role of MC4R signaling in somatic growth and reproduction in teleost fish.
RESULTS
agrp is Required for Normal Somatic Growth of Larval Zebrafish
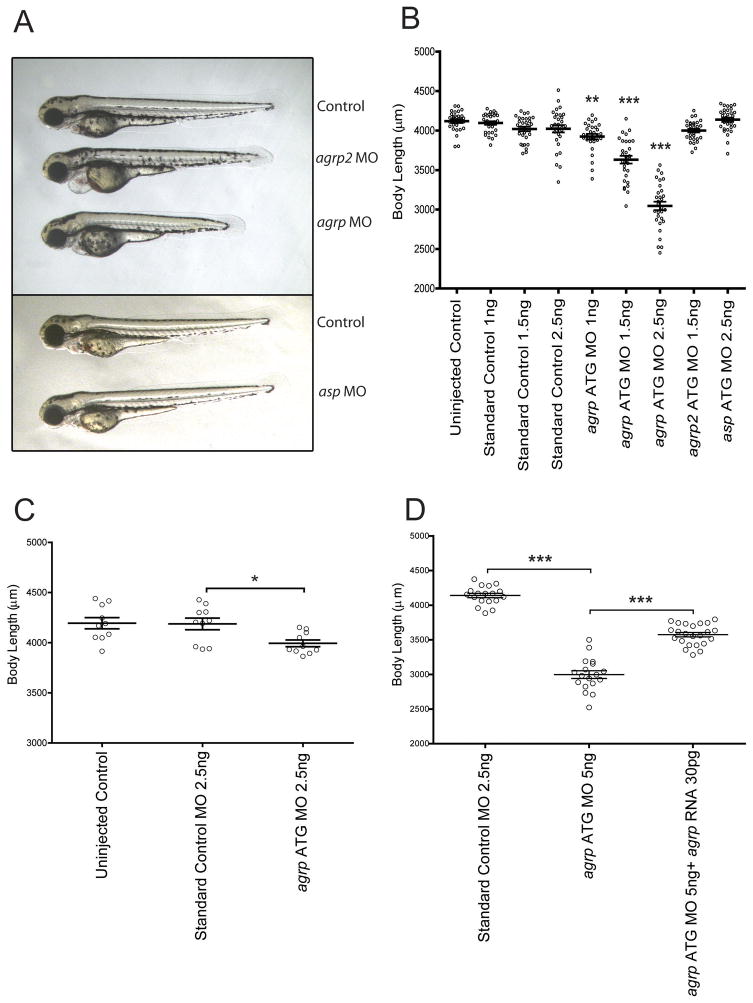
Since the endogenous antagonist of the MC4R, agouti-related protein (AgRP) is expressed as early as 1dpf in the fish (Song et al.), we designed agrp ATG targeting antisense morpholino oligonucleotides (MO) to block translation of the agrp gene in larval growth, allowing for increased MC4R signaling. MO are non-degradable synthetic nucleic acid analogues that can be designed to hybridize to complementary mRNA molecules to block translation or splicing (Summerton and Weller, 1997). Non-targeting standard control MO, and MO targeting the other agouti genes (asp and agrp2) were also injected. Dose-responsive suppression of somatic growth was clearly seen in agrp morphants at 3–5dpf (Figure 1A–B); a decrease in somite size, however was documented, but no decrease in somite number (not shown). An average body length of 4118 ± 23μm (uninjected controls) or 4023 ± 46μm (standard control MO injection) was reduced to 3045 ± 54μm by injection of 2.5 ng of agrp MO, for a 29% decrease in body length. MO blocking asp (4137 ± 27μm) or agrp2 (4000 ± 23μm) did not affect linear growth. We also designed two independent agrp MO to block splicing of the agrp mRNA. These MO were both demonstrated, using Q-PCR, to reduce full-length agrp mRNA levels by approximately 50%, and to produce a statistically significant reduction in length at 5 dpf (9–12% reduction, Figure S1).
Figure 1. agrp is Required for Normal Somatic Growth in Larval Zebrafish.
Representative fish at 3dpf following injection at day 0 with morpholino oligonucleotides designed to inhibit expression of each of the zebrafish agouti proteins (A). Body lengths (jaw to tail fin) of injected fish measured using a micrometer at 5dpf, showing a dose-responsive inhibition of growth following morpholino blockade of agrp expression (B). Body lengths corresponding to fish injected at day 0 were measured at 14 dpf, showing partial restoration of length (C). Body lengths at 5 dpf following injection of zygotes with 5 ng morpholino oligonucleotide indicated, plus 30pg capped agrp RNA, where indicated, showing partial rescue of growth (D). Bars indicates mean ± s.e.m. n= 30 (B), 10 (C), and 18–23 (D). Statistical significance tested by one way ANOVA followed by Tukey post test (*,p<0.05; **, p<0.01; ***, p<0.001). See also Figure S1.
Following MO injection and measurement at 5dpf, fish were maintained as described (Experimental Procedures). At 14dpf, the body length of 10 randomly chosen fish from each condition was measured again with a micrometer. Growth normally plateaus temporarily around 5dpf, and thus only slight further growth was seen in the uninjected or the control MO injected group (Figure 1C). However, at 14 dpf, agrp morphants receiving 2.5ng of MO catch up in length, from 3045 ± 54μm at 5dpf to 3993 ± 33μm at 14dpf, although they were still somewhat shorter compared with uninjected control (4193 ± 55μm) and 2.5ng standard control morphants (4187 ± 58μm). Thus, agrp MO injection does not cause a permanent developmental defect, since somatic growth catches up after inhibition of agrp expression by MO decays, around 3–7 days after injection.
Finally, we also demonstrated specificity using a rescue experiment. First, a construct was generated to produce agrp mRNA that was genetically altered to minimize the suppression of this mRNA by the agrp ATG blocking MO. Next, we co-injected 30pg 5′ capped zebrafish agrp mRNA with 2.5ng agrp MO oligo. Coinjection of 30pg agrp RNA produced a significant rescue from the growth suppression resulting from 2.5 ng agrp MO (Figure 1D). These studies further support the hypothesis that normal larval growth specifically requires agrp expression.
agrp Regulates Somatic Growth Via the mc4r
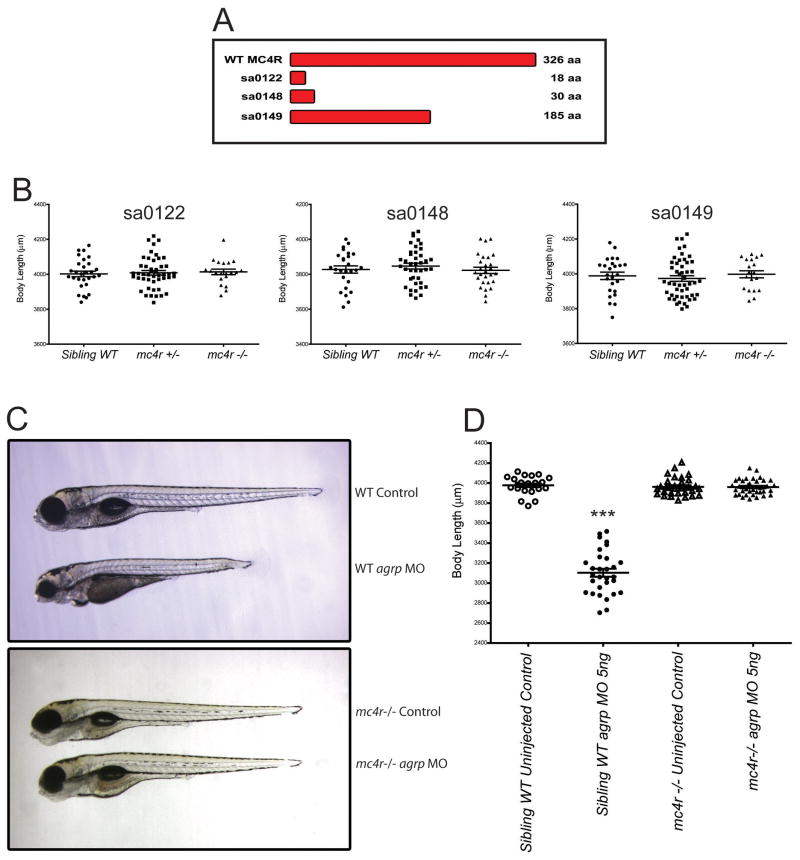
Zebrafish AgRP is an antagonist of 5 of the 6 zebrafish melanocortin receptors, with some specificity for the MC4R (Zhang et al., 2010). Several melanocortin receptor subtypes are expressed in the teleost CNS (Cerda-Reverter et al., 2003a; Cerda-Reverter et al., 2003b; Klovins et al., 2004). To determine if the MC4R is the pharmacological target for the inhibition of somatic growth by MO blockade of agrp, we obtained three zebrafish mc4r mutant strains from the Sanger Institute Zebrafish Mutation Project. Wild type zebrafish MC4R protein has 326 amino acids while each mc4r mutant carries a nonsense mutation resulting in premature translation termination, and expression of truncated proteins of 18, 30, or 185 amino acids; none of these truncated proteins could be expected to encode a functional GPCR protein (Figure 2A). As a control, we first examined the ability of mc4r loss to stimulate larval growth, since pharmacological blockade of the MC4R by agrp overexpression was shown to produce larger adult fish (Song and Cone, 2007). Heterozygous or homozygous loss of mc4r had no impact on length of fish at 5dpf (Figure 2B) in sa0122 (sibling WT: 4002 ± 15μM; heterozygous: 4008 ± 13μM; homozygous: 4013 ± 16μM), sa0148 (sibling WT: 3827 ± 20μM; heterozygous 3847 ± 16μM; homozygous 3822 ± 18μM) or sa0149 (sibling WT: 3988 ± 21μM; heterozygous 3973 ± 16μM; homozygous 3997 ± 20μM). Increased length resulting from homozygous loss of mc4r was detectable, however, as early as 42 days post-fertilization, and sustained in adult fish (Figure S2A–C).
Figure 2. mc4r is Required for Suppression of Somatic Growth by Morpholino Oligonucleotide Blockade of agrp.
Bar diagram showing the size of wild type and mutant single mc4r coding alleles in three lines identified by TILLING (Sanger Institute) (A). The maximal potential protein coding segment of each receptor mutant is sa0122: 18 aa; sa0148: 30 aa; sa0149: 185 aa. mc4r mutations do not affect early larval growth (B). Offspring from heterozygote matings of sa0122, sa0148 and sa0149 were measured at 5dpf, then genotyped by PCR. Numbers of fish analyzed in each group from left to right are 29, 47, 20 (sa0122), 26, 40, 27 (sa0148) and 25, 49, 19 (sa0149). Representative injected (5ng agrp MO) or uninjected control sa0149 fish at 5 dpf with genotype and experimental treatment indicated (C). Body lengths of fish treated as indicated (n=24–36) were measured with a micrometer at 5dpf, showing no effect of morpholino blockade of agrp on growth in the sa0149 mc4r −/− fish (D). Bars indicate mean ± s.e.m. Results were analyzed by one way ANOVA followed by Tukey post test. (***, p<0.001). See also Figures S2.
To test the role of the mc4r in growth inhibition 5ng agrp MO oligo was injected into offspring of matings between sa0149 MC4R +/− fish. Significant growth retardation was seen in sibling WT (3103 ± 40μM) compared with uninjected control fish (3978 ± 17μM). mc4r −/− fish, however appeared completely resistant to the growth suppressing effects of agrp MO, retaining their body length (3960 ± 13μM) in comparison with control group (3962 ± 16μM) (Figure 2C–D). MOs designed to block agrp splicing (Figure S1) were also non-functional in the mc4r −/− background (data not shown). If the consequence of agrp blockade is enhanced stimulation of the MC4R by the endogenous ligand, α-MSH, then reduction of the pomca preprohormone gene encoding α-MSH should also reduce the consequences of agrp blockade. Indeed, co-injection of a pomca MO with agrp MO blunted the inhibition of somatic growth by agrp MO alone (Figure S2D). Together, the data support the hypothesis that agrp has a specific role in the regulation of somatic growth as an antagonist of α-MSH mediated stimulation of MC4R signaling, and that the MC4R must be inhibited by AgRP during the larval period for the normal rate of growth to occur. Interestingly, we observed that high levels of agrp mRNA are already observed in the embryo at 1 dpf, as previously reported (Song et al., 2003), but that melanocortin receptor expression appears to increase more gradually from 1–3dpf (Figure S2E–F).
agrp Regulates Expression of Growth and Reproductive Hormones
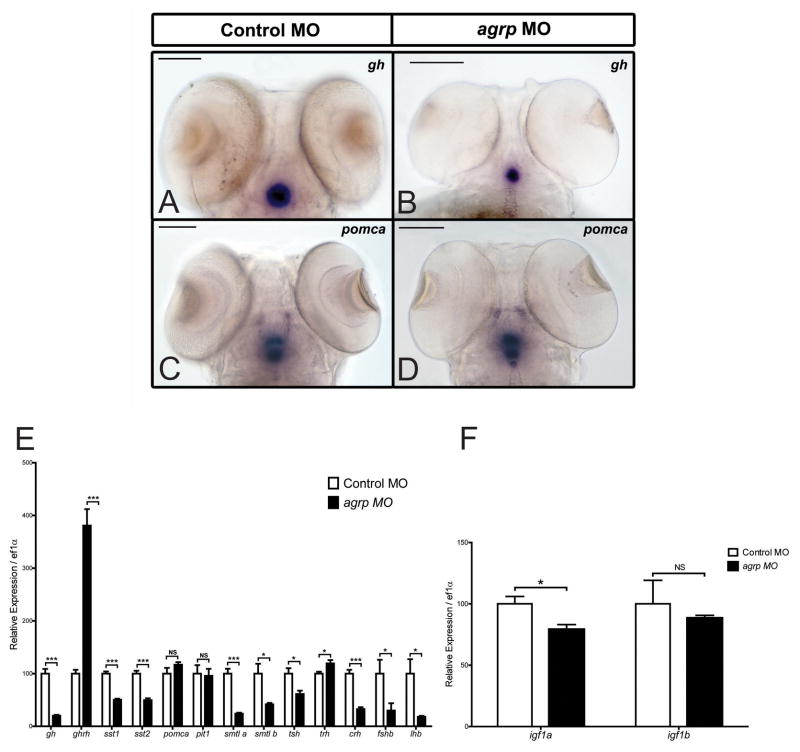
Growth hormone is a key factor stimulating somatic growth in teleosts. While absence of gh alone does not reduce larval growth, pit1 mutant zebrafish lacking growth hormone and prolactin exhibit severe dwarfism at 1 month of age (Nica et al., 2004). To identify the mechanism by which mc4r signaling regulates growth, expression of somatotropic and other pituitary hormones was examined. Whole mount in situ hybridization at 4dpf demonstrated a dramatic reduction of gh mRNA in pituitary (Figure 3A–B) following agrp MO injection. To determine if there was a general defect in pituitary gene expression, we examined expression of another pituitary preprohormone gene, proopiomelanocortin a (pomca, Figure 3C–D). pomca mRNA levels appeared unchanged. Next, we quantitated the relative expression at 4dpf of gh and other genes involved in somatic growth and pituitary function by PCR. gh appeared downregulated 4 fold, while ghrh was upregulated 3.8-fold, and both somatostatin genes were decreased approximately 50% (Figure 3E). The increase in hypothalamic ghrh and decrease in sst1 and sst2 gene expression argues that agrp blockade acts to block gh expression at the level of the pituitary, since the changes in ghrh and sst1/sst2 would represent normal neuroendocrine compensation in response to decreased gh expression. Similarly, while pituitary tsh was suppressed, hypothalamic trh expression was elevated, also arguing for the pituitary as a primary site of action. Pituitary development appeared normal, as levels of expression of pit1 and other pituitary genes such as pomca remained unchanged. As downstream mediators of the growth hormone pathway, insulin-like growth factors were also analyzed. One of the IGF genes, igf1a, was significantly reduced (Figure 3F). Interestingly, several other genes involved in neuroendocrine regulation were also altered specifically by agrp MO administration. For example, the reproductive hormones follicle stimulating hormone b (fshb), and luteinizing hormone b (lhb) all appeared suppressed by agrp MO treatment (Figure 3E).
Figure 3. agrp Regulates Expression of Multiple Pituitary Hormones in Zebrafish.
Whole mount in situ hybridization of gh (A–B) and pomca (C–D) in fish injected with 2.5ng standard control (A,C) or agrp MO oligonucleotides (B,D). Scale bars: 100μM. Relative expression levels of gh (growth hormone), ghrh (growth hormone releasing hormone), sst1 (somatostatin 1), sst2 (somatostatin 2), pomca (proopiomelanocortin a), pit1 (pituitary-specific positive transcription factor 1), sml a (somatolactin a), sml b, tsh (thyroid stimulating hormone), trh (thyrotropin releasing hormone), crh (corticotropin releasing hormone) fshb (follicle stimulating hormone), lhb (luteinizing hormone), igf1a (insulin-like growth factor 1a), and igf1b, were analyzed by Q-PCR in 30–40 4dpf fish injected with 2.5ng standard control or agrp MO oligonucleotides (E–F). mRNA expression was normalized to ef1a mRNA. Results were expressed as mean + s.e.m., and statistical analysis was done by unpaired t-test (*P<0.05;***P<0.001; NS: not significant). See also Figure S3.
To confirm the role of the mc4r in regulation of the growth hormone axis by agrp, we also examined expression of gh and pomca by whole mount in situ hybridization at 4dpf in mc4r −/− fish treated with control or agrp MO. First, we characterized baseline levels of pomca, pomcb, mc4r, agrp, gh, and ghrh in WT and mc4r −/− fish by Q-PCR; no significant differences were seen. In contrast to WT fish, no change in gh expression was seen in mc4r −/− fish treated with agrp MO (Figure S3).
AgRP-ir and POMC-ir Fibers Project to the Zebrafish Pituitary
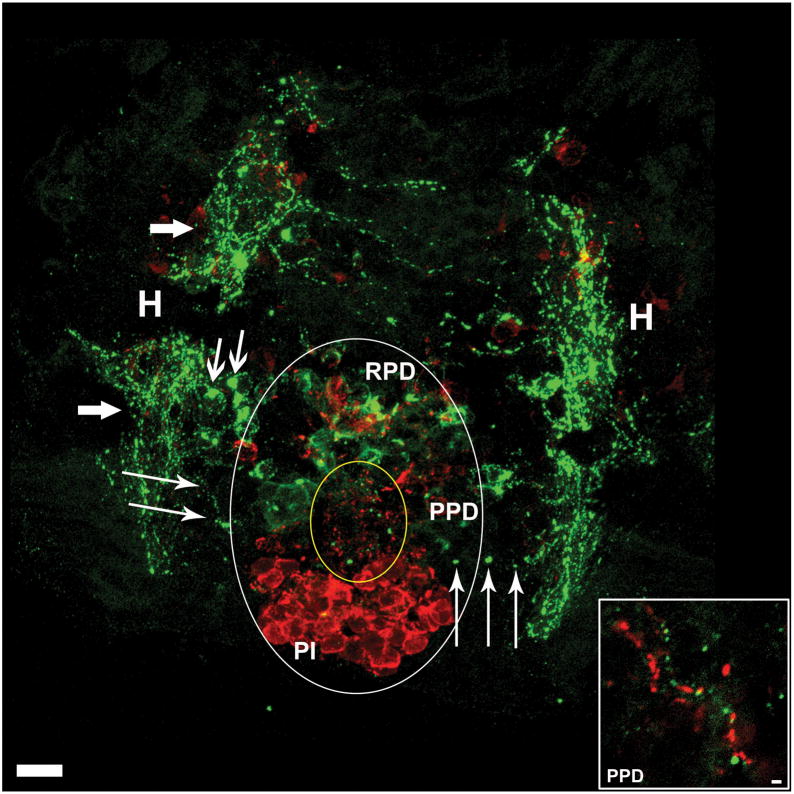
The ability of agrp to regulate multiple pituitary hormones implied a unique neuroendocrine role for these neurons, and we thus sought to further characterize AgRP and POMC neuroanatomy in the fish. Teleosts differ from mammals in hypothalamic-pituitary axis anatomy. The mammalian pituitary is functionally connected to the hypothalamus by the median eminence via a structure called the infundibular stem (pituitary stalk) (Low, 2008); in contrast, teleosts lack the hypothalamic-hypophysial-portal system and hypophysiotropic neurons project directly into the anterior pituitary (Janz, 2000). The zebrafish pituitary sits juxtaposed to the lateral tuberal nucleus (the ventral periventricular hypothalamus, proposed homologue to the arcuate nucleus), where AgRP is primarily expressed (Forlano and Cone, 2007). To determine the anatomical basis for direct regulation of gh lhb, fshb, and tsh by agrp, we sought to determine if AgRP-immunoreactive nerve fibers projected from the lateral tuberal nucleus to pituitary in the zebrafish. Double-labelled immunofluorescence was performed directly on brain sections from 5dpf zebrafish, staining for AgRP and α-MSH. Confocal imaging of a horizontal section clearly shows zebrafish AgRP-ir fibers (green), originating in hypothalamic fiber bundles, projecting from hypothalamus to the posterior and rostral pars distalis (RPD, PPD), while no fibers were seen in pars intermedia (PI, Figure 4). Putative pituitary melanotrope cell bodies staining positively for α-MSH (red) dominate the PI. While hypothalamic α-MSH expressing POMC cell bodies, seen rostrally, are largely out of focus in this image, α-MSH-IR fibers are readily visible in the RPD and PPD. The AgRP-ir in the RPD appears to result from dense fiber bundles encircling cells, rather than staining of pituicytes. The inset is a magnification of a region of the PPD showing parallel AgRP-ir and α-MSH-ir neuronal fibers. These data indicate that hypothalamic AgRP and α-MSH fibers project to multiple sub-regions in the pituitary where many hormones are synthesized, including growth hormone, gonadotropins (FSH and LH) prolactin and somatolactin (Kasper et al., 2006). Direct action of AgRP and/or POMC peptides such as α-MSH in pituitary would likely require expression of melanocortin receptors in this organ. Using RT-PCR with tissues from adult zebrafish, we identified zebrafish mc4r RNA expression in the pituitary gland. (Figure S4).
Figure 4. Hypothalamic AgRP and α-MSH Expressing Neurons Project to the Pituitary.
Horizontal view of larval zebrafish brain at 5dpf illustrating the pituitary and underlying hypothalamus. Large white oval outlines the extent of the pituitary, and small yellow oval indicates the anterior zone equivalent containing dense α-MSH immunoreactive (ir) fibers (red) and AgRP-ir fibers (green). Large arrows indicate hypothalamic AgRP-ir fiber bundles, medium arrows indicate hypothalamic AgRP-ir cell bodies, and thin arrows indicate AgRP-ir fibers projecting from hypothalamus into the pituitary. Inset is an enlargement from the PPD showing parallel AgRP-ir and α-MSH-ir neuronal fibers PI, pars intermedia; PPD, proximal pars distalis; RPD, rostral pars distalis; H, hypothalamus. Scale bars: main image =10μm, inset = 1μm.
DISCUSSION
The many ascending hypothalamic AgRP and POMC projections in the zebrafish exhibit conserved fiber pathways with their mammalian counterparts (Forlano and Cone, 2007). However, the data here represent a major neuroanatomical and functional departure for the teleost melanocortin system. In mammals, the melanocortin circuitry integrates information about energy stores with a subset of endocrine axes by serving as a leptin-responsive input to hypophysiotropic neurons in the hypothalamus, which in turn regulate pituitary hormone release (Cone, 2005; Tao, 2010). For example, the mammalian melanocortin circuits regulate the thyroid axis (Fekete et al., 2000a; Nillni et al., 2000). Using leptin-mediated regulation of the thyroid axis as an example, data show that TRH neurons in the paraventricular nucleus of the hypothalamus receive direct projections from arcuate POMC and AgRP neurons (Fekete et al., 2000b; Fekete et al., 2000c; Harris et al., 2001; Perello et al., 2006; Toni et al., 1990). Leptin acts directly on these arcuate neurons (Bates et al., 2004; Elmquist et al., 1998; Hubschle et al., 2001; Perello et al., 2006) to control the release α-MSH and AgRP thus regulating the level of activity of the melanocortin-4 receptor (MC4-R), and perhaps to some extent directly on the TRH neurons themselves (Ghamari-Langroudi et al., 2010), to regulate expression and release of TRH to regulate activity of the thyroid axis. In contrast, mammalian melanocortin circuits have little impact on the reproductive axis (Irani et al., 2005), and have small effects on growth that do not appear to result from measurable increases, relative to normal individuals, in growth hormone or IGF expression (Martinelli et al., 2011). Some data suggests that the chronic blockade of the MC4R in the agouti mouse results in decreased hypothalamic somatostatin levels (Martin et al., 2006). Thus, the available data thus far indicate that in mammals, melanocortin circuits regulate endocrine function via control of the hypothalamic releasing hormone neurons.
In stark contrast to what is known in mammalian systems, data presented here demonstrate that the larval teleost POMC and AgRP neurons are hypophysiotropic, project to the pituitary, and directly and coordinately regulate expression of multiple endocrine axes. While the data indicate that MC4R mRNA is found in the pituitary, additional work will be needed to determine if functional MC4R is expressed in pituicytes such as the somatotropes, and regulates hormones such as GH in direct response to AgRP and α-MSH released from hypophysiotropic AgRP and POMC neurons. During the first 5 days post fertilization, zebrafish larvae do not feed, but rather acquire nutrients from the yolk sac. During this period, mutations in the MC4R do not measurably increase growth rate, which might be explained by the relatively high ratio of agrp/pomca expressed from 1–3dpf producing chronic blockade of MC4R signaling (Figure S3E–F). In contrast, suppression of agrp expression reduces growth rate in a MC4R-dependent manner during this period. These data imply that MC4R suppression by agrp is required for the maximal rate of growth during this period, allowing rapid maturation and thus perhaps reducing predation. In contrast, at some point after feeding behavior begins, during a period when growth and food intake can be regulated in response to environmental conditions, MC4R is no longer fully suppressed, and reduced MC4R activity, such as seen in mutant lines, can increase growth rate.
Taken together, the data provide a mechanistic basis for understanding natural variation in teleost growth and reproduction. Male size polymorphisms have been reported in many teleost species including plainfin midshipman (Brantley and Bass, 1994), blenny (Oliveira et al., 2001), guppy (Tripathi et al., 2009), and swordtail platyfish (Xiphophorus nigrensis and multilineatus). The data presented here provide an anatomical and functional basis for the co-regulation of growth and reproductive behavior seen in MC4R variants encoded by the P locus (Lampert et al., 2010). The central melanocortin system is thus a unique substrate for coordinate regulation of endocrine function, and feeding and reproductive behavior. This, in turn, suggests that alterations in melanocortin signaling may play a role in the evolution of the diverse array of reproductive, growth, and feeding strategies observed in teleosts. Finally, in mammals endocrine function is coordinated with energy state in large part via the action of the adipostatic hormone leptin (Ahima et al., 1996; Chehab et al., 1996) acting on independent hormone target sites in the hypothalamus that project to and regulate hypophysiotropic neurons. Leptin is poorly conserved in non-mammalian vertebrates, and may not even exist in birds (Copeland et al., 2011); this, along with the unique ability of the central melanocortin circuits in teleosts to respond to energy state (Song et al., 2003) and coordinately regulate endocrine function also implies significant differences in energy homeostasis between mammalian and non-mammalian vertebrates.
EXPERIMENTAL PROCEDURES
Experimental Animals
Wild type Tab 14 or AB strain zebrafish were raised and bred at 26–28 °C, with 14 hour light, 10 hour dark cycle. Larval stage was determined according to Kimmel et al (Kimmel et al., 1995). Fish aged from 5 dpf to 10 dpf were fed twice a day with rotifers and baby powder, fish from 10 dpf to 15 dpf were fed with rotifer supplemented with uncapsulated brine shrimp, and fish from 15 dpf to 1 month or older were fed with uncapsulated brine shrimp. For adult fish, food was prepared by mixing 4 parts of tropical flakes (Aquatic Eco-systems, Inc. Apopka, FL, USA) and 1 part of brine shrimp (Brine Shrimp Direct, Ogden, UT, USA) in system water. mc4r −/− mutant strains were obtained from the Sanger Institute Zebrafish Mutation Project, and genotyped as described (Supplemental Information). All studies were approved by the animal care and use committee of Vanderbilt University.
RNA Extraction, cDNA Synthesis and Real Time Quantitative PCR (Q-PCR)
Embryos were homogenized in lysis buffer with a sonic dismembrator (model 100, Fisher Scientific, Pittsburgh, PA, USA). Total RNA was extracted using an RNeasy mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. To remove genomic DNA, On-column DNase Digestion was performed using an RNase free DNase Set (Qiagen, Valencia, CA, USA). 1μg of purified total RNA was reverse transcribed with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Q-PCR primers were designed by Beacon Designer 7.0 (Premier Biosoft International, Palo Alto, CA, USA) to minimize primer self-dimerization, and primer sequences are provided in the Supplemental Information. Q-PCRs were performed using 2μl of cDNA (20ng) as template, 5 pmol of each of forward and reverse primers, 2X Power SYBR PCR mix (Applied Biosystems, Carlsbad, CA, USA) with nuclease free water (Promega, Madison, WI, USA) to make the final volume to 20mL in a 96 well plate (Bioexpress, Kaysville, UT, USA). Q-PCRs were performed using an Mx3000PTM (Stratagene, Santa Clara, CA, USA). The PCR cycle was performed according to manufacturer’s instructions with initial denaturation at 95 °C for 10 min, followed by 45 cycles of 95 °C 20 sec, 60°C 60 sec. At the end of the cycles, melting curves of the products were verified for the specificity of PCR products. A standard curve with serial dilutions of cDNA sample was performed on each plate. All measurements were performed in duplicate and prism 5.0 was used for the interpretation and analysis of data.
Whole Mount In Situ Hybridization
To generate antisense digoxigenin (Dig)-labeled cRNA probes, pCr4-TOPO plasmids containing full length gh1 and pomca sequences (see Supplemental Information) were linearized by digestion with Not I and subjected to in vitro transcription with T3 RNA polymerase. For sense Dig-labeled cRNA probe, plasmids were linearized by digestion with Spe I and subjected to in vitro transcription with T7 RNA polymerase according to the manufacturer’s protocol (Roche, Indianapolis, IN, USA). Zebrafish embryos at different developmental stages were collected, manually dechorionated and fixed in 4% paraformaldehyde in PBS at room temperature for 3–5 hours. Whole mount in situ hybridization was performed as described previously (Westerfield, 2000). Briefly, fixed embryos were treated with −20 °C methanol and rehydrated with a series of descending methanol concentrations (75%, 50% and 25%) in PBS. They were then washed with PBS and treated with proteinase K (Fermentas, Glen Burnie, Maryland, MD, USA) for 8 minutes at room temperature at a concentration of 10 μg/ml in PBS up to 24 hpf, 20 μg/ml from 24 hpf to 72 hpf and 50μg/ml up to 15 dpf. Embryos were refixed with 4% paraformaldehyde in PBS at room temperature for 20 minutes, washed 5 times with PBS, prehybridized with hybridization buffer (50% formamide, 5X SSC, 50μg/ml heparin (Sigma, St. Louis, MO, USA), 500μg/ml tRNA (Roche, Indianapolis, IN, USA), 0.1% Tween-20 and 9.2 mM Citric Acid (pH.6.0) at 65 °C for 3 hrs, then probed with either antisense or sense Dig-labeled probe at 65 °C overnight at 500 ng/ml in hybridization buffer. Dig-labeled cRNA probes were detected with 1:2000 diluted alkaline phosphatase conjugated anti-digoxigenin antibody (Roche, Indianapolis, IN, USA) in 2% BMB (Roche, Indianapolis, IN, USA), 20% lamb serum (Gibco BRL, Carlsbad, CA, USA) in MAB (100mM Maleic Acid, 150 mM NaCl, 0.1% Tween-20, pH7.5) at 4 °C overnight, followed by staining with NBT/BCIP solution (Roche, Indianapolis, IN, USA) at room temperature for 2–5 hours. After PBS washing, methanol was applied to the stained embryos to remove the nonspecific stain, and refixed in 4% paraformaldehyde in PBS. The embryos were mounted in 100% glycerol and pictures were taken by AxionVision (Ver3.1) software with a StemiSV11 Dissecting Microscope (Carl Zeiss INC,).
Morpholino Oligonucleotides Injection, RNA Rescue Experiment and Body Length Measurement
Morpholino oligonucleotides, prepared as described (Supplemental Information) were dissolved in nuclease-free water and stored in −20°C as 1mM stock. Serial dilutions were made using nuclease-free water to 0.1, 0.2, 0.3, 0.4mM working solution with 20% Phenol Red (Sigma, St. Louis, MO, USA. 0.5% in DPBS, sterile filtered, endotoxin tested). Before the injection, MOs were denatured at 65 °C for 5min and quickly spun to avoid the formation of aggregates. 3–5 μL was loaded in a micro-injection machine and embryos at one or two cell stages were injected with1–2 nL of a solution containing antisense targeting-morpholino or standard control oligo. Each MO oligo injection was repeated at least three times and doses were adjusted to optimize the phenotype-to-toxicity ratio. Following morpholino injections, embryos were raised in egg water, changed daily, under standard light/dark cycle up to 6 days post fertilization. Dead embryos were excluded at 1dpf. Embryos were assayed for whole mount in situ hybridization and qRT-PCR at 4 dpf. Linear body length (forehead to tail fin) was determined using a micrometer at 5dpf, 14dpf, and 42dpf. Embryos were mounted in 2.5% methyl cellulose and images were taken by AxionVision (Ver3.1) software with a Lumar V12 Stereo Microscope (Carl Zeiss).
Full length agrp including 5′ UTR sequence for mRNA rescue was cloned into PCS2+ vector, which contains a 5′ SP6 promoter and 3′ SV40 polyA tail. To make 5′ capped zebrafish agrp RNA, plasmids was linearized by digestion with Not I and subjected to in vitro transcription with SP6 RNA polymerase in presence of 0.5mM Ribo m7G Cap Analog (Promega, Madison, MI, USA). RNA was purified by mini Quick Spin™ Column (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions. agrp ATG antisense MO was injected at 0.3mM with or without 30ng/uL capped agrp RNA. Embryos were raised at standard dark/light cycle and body length was measured at 5dpf.
Immunocytochemistry
Zebrafish larvae were anesthetized in MS222 (tricaine methanesulfonate; Sigma, St. Louis, MO, USA) on ice and fixed whole with teleost Ringer’s solution followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.2) for 1 hour at RT. After fixation, larvae were washed in PB and cryoprotected in 30% sucrose in PB overnight at 4°C. And then were embedded in Tissue-TeK OCT medium (Sakura Finetek, Torrance, CA, USA) in Tissue-Tek intermediate cryomolds and stored at −80°C until sectioned on a cryostat in the transverse, sagittal, or horizontal plane at 16 μm and collected onto Superfrost Plus slides (Fisher Scientific, Fair Lawn, NJ, USA). The immunocytochemical labeling protocol followed Forlano and Cone (Forlano and Cone, 2007) and slides were coverslipped with SlowFade Gold with DAPI (Molecular Probes, Carlsbad, CA, USA) nuclear counterstain to provide cytoarchitectonic detail. Micrographs were taken on a Zeiss LSM 710 META Inverted Confocal Microscope.
Supplementary Material
Highlights.
AgRP and POMC neurons are hypophysiotropic in the teleost
Melanocortin signaling co-regulates multiple pituitary hormones in the teleost
MC4R is suppressed by endogenous AgRP to maximize somatic growth in 0–5dpf larvae
Provides a mechanistic basis for evolution of variant growth/reproductive strategies
Acknowledgments
Supported by NIH grant RO1DK075721 (R.D.C.). The authors would like to thank Drs. Sam Wells and Bob Matthews for help with confocal microscopy.
Footnotes
The authors declare no conflict of interest.
Supplemental Information includes four figures and supplemental experimental procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Ahima R, Prabakaran D, Mantzoros C, Qu D, Lowell B, TMF, Flier S. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MG. LRb-STAT3 Signaling Is Required for the Neuroendocrine Regulation of Energy Expenditure by Leptin. Diabetes. 2004;53:3067–3073. doi: 10.2337/diabetes.53.12.3067. [DOI] [PubMed] [Google Scholar]
- Brantley RK, Bass AH. Alternative Male Spawning Tactics and Acoustic Signals in the Plainfin Midshipman Fish Porichthys notatus Girard (Teleostei, Batrachoididae) Ethology. 1994;96:213–232. [Google Scholar]
- Cerda-Reverter JM, Ling MK, Schioth HB, Peter RE. Molecular cloning, characterization and brain mapping of the melanocortin 5 receptor in the goldfish. J Neurochem. 2003a;87:1354–1367. doi: 10.1046/j.1471-4159.2003.02107.x. [DOI] [PubMed] [Google Scholar]
- Cerda-Reverter JM, Peter RE. Endogenous melanocortin antagonist in fish: structure, brain mapping, and regulation by fasting of the goldfish agouti-related protein gene. Endocrinology. 2003;144:4552–4561. doi: 10.1210/en.2003-0453. [DOI] [PubMed] [Google Scholar]
- Cerda-Reverter JM, Ringholm A, Schioth HB, Peter RE. Molecular cloning, pharmacological characterization, and brain mapping of the melanocortin 4 receptor in the goldfish: involvement in the control of food intake. Endocrinology. 2003b;144:2336–2349. doi: 10.1210/en.2002-0213. [DOI] [PubMed] [Google Scholar]
- Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Copeland DL, Duff RJ, Liu Q, Prokop J, Londraville RL. Leptin in teleost fishes: an argument for comparative study. Front Physiol. 2011;2:26. doi: 10.3389/fphys.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, CB, RSA, JSF, CBS Distribution of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- Fekete C, Legradi G, Mihaly E, Huang Q-H, Tatro JB, Rand WM, Emerson CH, Lechan RM. α-melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci. 2000a;20:1550–1558. doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Legradi G, Mihaly E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM. alpha-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci. 2000b;20:1550–1558. doi: 10.1523/JNEUROSCI.20-04-01550.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Mihaly E, Luo L-G, Kelly J, Clausen JT, Mao Q, Rand WM, Moss LG, Kuhar M, Emerson, et al. Association of Cocaine- and Amphetamine-Regulated Transcript-Immunoreactive Elements with Thyrotropin-Releasing Hormone-Synthesizing Neurons in the Hypothalamic Paraventricular Nucleus and Its Role in the Regulation of the Hypothalamic-Pituitary-Thyroid Axis during Fasting. J Neurosci. 2000c;20:9224–9234. doi: 10.1523/JNEUROSCI.20-24-09224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Cone RD. Conserved neurochemical pathways involved in hypothalamic control of energy homeostasis. J Comp Neurol. 2007;505:235–248. doi: 10.1002/cne.21447. [DOI] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Vella KR, Srisai D, Sugrue ML, Hollenberg AN, Cone RD. Regulation of thyrotropin-releasing hormone-expressing neurons in paraventricular nucleus of the hypothalamus by signals of adiposity. Mol Endocrinol. 2010;24:2366–2381. doi: 10.1210/me.2010-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M, Shuttre JR, Sarmiento U, Sarosi I, Stark KL. Overexpression of Agrt leads to obesity in transgenic mice. Nature Genetics. 1997;17:273–274. doi: 10.1038/ng1197-273. [DOI] [PubMed] [Google Scholar]
- Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjoorbaek C, Elmquist JK, Flier JS, Hollenberg AN. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest. 2001;107:111–120. doi: 10.1172/JCI10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W. Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci. 2001;21:2413–2424. doi: 10.1523/JNEUROSCI.21-07-02413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Irani BG, Xiang Z, Moore MC, Mandel RJ, Haskell-Luevano C. Voluntary exercise delays monogenetic obesity and overcomes reproductive dysfunction of the melanocortin-4 receptor knockout mouse. Biochem Biophys Res Commun. 2005;326:638–644. doi: 10.1016/j.bbrc.2004.11.084. [DOI] [PubMed] [Google Scholar]
- Janz DM. Endocrine System. In: Ostrander GK, editor. The Laboratory Fish. Boston: Academic Press; 2000. pp. 190–191. [Google Scholar]
- Kallman KD, Borkoski V. A Sex-Linked Gene Controlling the Onset of Sexual Maturity in Female and Male Platyfish (XIPHOPHORUS MACULATUS), Fecundity in Females and Adult Size in Males. Genetics. 1978;89:79–119. doi: 10.1093/genetics/89.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper RS, Shved N, Takahashi A, Reinecke M, Eppler E. A systematic immunohistochemical survey of the distribution patterns of GH, prolactin, somatolactin, beta-TSH, beta-FSH, beta-LH, ACTH, and alpha-MSH in the adenohypophysis of Oreochromis niloticus, the Nile tilapia. Cell Tissue Res. 2006;325:303–313. doi: 10.1007/s00441-005-0119-7. [DOI] [PubMed] [Google Scholar]
- Kimmel C, Ballard W, Kimmel S, Ullmann B, Schilling T. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Klovins J, Haitina T, Fridmanis D, Kilianova Z, Kapa I, Fredriksson R, Gallo-Payet N, Schioth HB. The melanocortin system in Fugu: determination of POMC/AGRP/MCR gene repertoire and synteny, as well as pharmacology and anatomical distribution of the MCRs. Mol Biol Evol. 2004;21:563–579. doi: 10.1093/molbev/msh050. [DOI] [PubMed] [Google Scholar]
- Lampert KP, Schmidt C, Fischer P, Volff JN, Hoffmann C, Muck J, Lohse MJ, Ryan MJ, Schartl M. Determination of onset of sexual maturation and mating behavior by melanocortin receptor 4 polymorphisms. Curr Biol. 2010;20:1729–1734. doi: 10.1016/j.cub.2010.08.029. [DOI] [PubMed] [Google Scholar]
- Low MJ. Neuroendocrinology. In: Kronenberg HM, Melmed S, Polonsky KS, Larsen PR, editors. Williams Textbook of Endocrinology. Philadelphia: Saunders Elsevier; 2008. pp. 85–154. [Google Scholar]
- Martin NM, Houston PA, Patterson M, Sajedi A, Carmignac DF, Ghatei MA, Bloom SR, Small CJ. Abnormalities of the somatotrophic axis in the obese agouti mouse. Int J Obes (Lond) 2006;30:430–438. doi: 10.1038/sj.ijo.0803076. [DOI] [PubMed] [Google Scholar]
- Martinelli CE, Keogh JM, Greenfield JR, Henning E, van der Klaauw AA, Blackwood A, O’Rahilly S, Roelfsema F, Camacho-Hubner C, Pijl H, Farooqi IS. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab. 2011;96:E181–188. doi: 10.1210/jc.2010-1369. [DOI] [PubMed] [Google Scholar]
- Nica G, Herzog W, Sonntag C, Hammerschmidt M. Zebrafish pit1 mutants lack three pituitary cell types and develop severe dwarfism. Mol Endocrinol. 2004;18:1196–1209. doi: 10.1210/me.2003-0377. [DOI] [PubMed] [Google Scholar]
- Nillni EA, Vaslet C, Harris M, Hollenberg A, Bjorbaek C, Flier JS. Leptin regulates prothyrotropin-releasing hormone biosynthesis. J Biol Chem. 2000;275:36124–36133. doi: 10.1074/jbc.M003549200. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Carneiro LA, Canario AV, Grober MS. Effects of androgens on social behavior and morphology of alternative reproductive males of the Azorean rock-pool blenny. Horm Behav. 2001;39:157–166. doi: 10.1006/hbeh.2001.1643. [DOI] [PubMed] [Google Scholar]
- Perello M, Stuart RC, Nillni EA. The Role of Intracerebroventricular Administration of Leptin in the Stimulation of Prothyrotropin Releasing Hormone Neurons in the Hypothalamic Paraventricular Nucleus. Endocrinology. 2006;147:3296–3306. doi: 10.1210/en.2005-1533. [DOI] [PubMed] [Google Scholar]
- Schjolden J, Schioth HB, Larhammar D, Winberg S, Larson ET. Melanocortin peptides affect the motivation to feed in rainbow trout (Oncorhynchus mykiss) Gen Comp Endocrinol. 2009;160:134–138. doi: 10.1016/j.ygcen.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Song Y, Cone RD. Creation of a genetic model of obesity in a teleost. FASEB J. 2007;21:2042–2049. doi: 10.1096/fj.06-7503com. [DOI] [PubMed] [Google Scholar]
- Song Y, Golling G, Thacker TL, Cone RD. Agouti-related protein (AGRP) is conserved and regulated by metabolic state in the zebrafish, Danio rerio. Endocrine. 2003;22:257–265. doi: 10.1385/ENDO:22:3:257. [DOI] [PubMed] [Google Scholar]
- Summerton J, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni R, Jackson IM, Lechan RM. Neuropeptide-Y-immunoreactive innervation of thyrotropin-releasing hormone-synthesizing neurons in the rat hypothalamic paraventricular nucleus. Endocrinol. 1990;126:2444–2453. doi: 10.1210/endo-126-5-2444. [DOI] [PubMed] [Google Scholar]
- Tripathi N, Hoffmann M, Willing EM, Lanz C, Weigel D, Dreyer C. Genetic linkage map of the guppy, Poecilia reticulata, and quantitative trait loci analysis of male size and colour variation. Proc Biol Sci. 2009;276:2195–2208. doi: 10.1098/rspb.2008.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nature Genetics. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- Westerfield M. A guide for the laboratory use of zebrafish. Eugene, OR: University of Oregon Press; 2000. The Zebrafish Book. [Google Scholar]
- Yeo GSH, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nature Genetics. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- Zhang C, Song Y, Thompson DA, Madonna MA, Millhauser GL, Toro S, Varga Z, Westerfield M, Gamse J, Chen W, Cone RD. Pineal-specific agouti protein regulates teleost background adaptation. Proc Natl Acad Sci U S A. 2010;107:20164–20171. doi: 10.1073/pnas.1014941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.