Abstract
Objectives
This study was designed to assess the clinical course and to identify risk factors for life-threatening events in patients with long-QT syndrome (LQTS) with normal corrected QT (QTc) intervals.
Background
Current data regarding the outcome of patients with concealed LQTS are limited.
Methods
Clinical and genetic risk factors for aborted cardiac arrest (ACA) or sudden cardiac death (SCD) from birth through age 40 years were examined in 3,386 genotyped subjects from 7 multinational LQTS registries, categorized as LQTS with normal-range QTc (≤440 ms [n = 469]), LQTS with prolonged QTc interval (>440 ms [n = 1,392]), and unaffected family members (genotyped negative with ≤440 ms [n = 1,525]).
Results
The cumulative probability of ACA or SCD in patients with LQTS with normal-range QTc intervals (4%) was significantly lower than in those with prolonged QTc intervals (15%) (p < 0.001) but higher than in unaffected family members (0.4%) (p < 0.001). Risk factors ACA or SCD in patients with normal-range QTc intervals included mutation characteristics (transmembrane-missense vs. nontransmembrane or nonmissense mutations: hazard ratio: 6.32; p = 0.006) and the LQTS genotypes (LQTS type 1:LQTS type 2, hazard ratio: 9.88; p = 0.03; LQTS type 3:LQTS type 2, hazard ratio: 8.04; p = 0.07), whereas clinical factors, including sex and QTc duration, were associated with a significant increase in the risk for ACA or SCD only in patients with prolonged QTc intervals (female age >13 years, hazard ratio: 1.90; p = 0.002; QTc duration, 8% risk increase per 10-ms increment; p = 0.002).
Conclusions
Genotype-confirmed patients with concealed LQTS make up about 25% of the at-risk LQTS population. Genetic data, including information regarding mutation characteristics and the LQTS genotype, identify increased risk for ACA or SCD in this overall lower risk LQTS subgroup.
Keywords: corrected QT interval, long-QT syndrome, sudden cardiac death
Congenital long-QT syndrome (LQTS) is an inherited channelopathy characterized by a prolonged corrected QT interval (QTc) at rest that is associated with an increased predisposition for polymorphic ventricular arrhythmias and sudden cardiac death (SCD) in young subjects without structural heart disease (1). To date, more than 500 mutations have been identified in 12 LQTS-susceptibility genes, with the long-QT syndrome type 1 (LQT1), long-QT syndrome type 2 (LQT2), and long-QT syndrome type 3 (LQT3) genotypes constituting more than 95% of genotype-positive LQTS and approximately 75% of all LQTS (2). Risk assessment in affected patients with LQTS relies primarily on a constellation of electrocardiographic (ECG) and clinical factors, including QTc interval and age-sex interactions (3–6). In addition, there is increasing evidence that genetic information and the molecular and cellular properties of the LQTS-causative mutation may identify subjects with increased risk for cardiac events (7–10). Despite these recent advances, however, currently there are limited data regarding the clinical course and risk factors for life-threatening events in patients with LQTS with normal resting QTc values, so-called silent mutation carriers, concealed LQTS, or normal–QT interval LQTS.
In the present study we used combined data from 7 national LQTS registries to: 1) compare the clinical courses of patients with LQTS and normal-range QTc intervals to those of patients with prolonged QTc intervals and of genotype-negative unaffected family members; and 2) identify specific clinical and genetic risk factors for life-threatening cardiac events in patients with LQTS with normal-range QTc intervals.
Methods
Study population
The study population comprised 3,386 genotyped subjects drawn from the Rochester, New York, enrolling center (center 1) of the International LQTS Registry (n = 2,630), the Netherlands LQTS Registry (n = 391), and the Japanese LQTS Registry (n = 205), as well as from data submitted by other investigators specifically for this collaborative mutation analysis project from Denmark (n = 90), Italy (n = 28), Israel (n = 25), and Sweden (n = 17). Patients were derived from 552 proband-identified KCNQ1 (LQT1), KCNH2 (LQT2), and SCN5A (LQT3) families. The proband in each family had otherwise unexplained, diagnostic QTc prolongation or experienced LQTS-related symptoms. Patients were excluded from the study if they had: 1) >1 LQTS identified mutation (n = 70); 2) Jervell and Lange-Nielsen syndrome with deafness and 2 KCNQ1 mutations or 1 known KCNQ1 mutation and congenital deafness (n = 2); and 3) no identified mutation on genetic testing with prolonged QTc interval (>440 ms [n = 428]).
Data collection and end point
Routine clinical and rest ECG parameters were acquired at the time of enrollment in each of the registries. Measured parameters on the first recorded electrocardiogram included QT and R-R intervals in milliseconds, with QT interval corrected for heart rate using Bazett’s (11) formula. Clinical data were collected on prospectively designed forms with information on demographic characteristics, personal and family medical histories, ECG findings, therapies, and events during long-term follow-up. Data common to all LQTS registries involving genetically tested subjects were electronically merged into a common database for the present study. In addition, information regarding QT interval–prolonging medications and triggers for cardiac events was collected through a specific questionnaire for patients enrolled the U.S. portion of the registry.
The primary end point of the study was the occurrence of a first life-threatening cardiac event, comprising aborted cardiac arrest (ACA; requiring external defibrillation as part of the resuscitation or internal defibrillation in patients with implantable cardioverter-defibrillators) or LQTS-related SCD (abrupt in onset without evident cause, if witnessed, or death that was not explained by any other cause if it occurred in a nonwitnessed setting such as sleep). In the multivariate models, follow-up was censored at age 41 years to avoid the influence of coronary disease on the occurrence of cardiac events. We also evaluated a secondary end point that included the occurrence of a first cardiac event of any type during follow-up (comprising syncope [defined as transient loss of consciousness that was abrupt in onset and offset], ACA, or SCD).
Phenotype characterization
For the purpose of this study, the QTc interval was categorized as normal range (≤440 ms) or prolonged (>440 ms) according to accepted criteria for the phenotypic definition of LQTS (12). Using this definition, the study population were categorized into 3 genotype and QTc subgroups: 1) LQTS with normal-range QTc interval (n = 469), comprising patients identified to have LQT1 to LQT3 mutations with QTc intervals ≤440 ms; 2) LQTS with prolonged QTc interval (n = 1,392), comprising patients with LQT1 to LQT3 mutations with QTc intervals >440 ms; and 3) unaffected family members (n = 1,525), comprising registry subjects from genotype-positive proband-identified families who were genetically tested and found to be negative for the LQTS-associated mutation, with QTc intervals ≤440 ms (i.e., genetically and phenotypically unaffected family members).
Genotype characterization
The KCNQ1, KCNH2, and SCN5A mutations were identified with the use of standard genetic tests performed in academic molecular genetics laboratories, including the Functional Genomics Center, University of Rochester Medical Center, Rochester, New York; Baylor College of Medicine, Houston, Texas; Windland Smith Rice Sudden Death Genomics Laboratory, Mayo Clinic, Rochester, Minnesota; Boston Children’s Hospital, Boston, Massachusetts; the Laboratory of Molecular Genetics, National Cardiovascular Center, Suita, Japan; the Department of Clinical Genetics, Academic Medical Center, Amsterdam, the Netherlands; and the Molecular Cardiology Laboratory, Policlinico S. Matteo and University of Pavia, Pavia, Italy.
Genetic alterations of the amino acid sequence were characterized by location and by the type of the specific mutation. The transmembrane region of each of the 3 LQTS channels was defined as: 1) amino acid residues from 120 through 355 in the KCNQ1-encoded Kv7.1 channel (S1 to S6 region); 2) amino acid residues from 398 through 657 (S1 to S6 region) in the KCNH2-encoded Kv11.1 channel; and 3) amino acid residues 129 through 417, 713 through 940, 1201 through 1470, and 1523 through 1740 in the SCN5A-encoded Nav1.5 channel (13). On the basis of prior studies that demonstrated the functional and clinical importance of missense mutations that are located in the transmembrane region of these LQTS-associated channels (9,10), mutation categories were pre-specified in the primary analysis as transmembrane-missense (mutations of the missense type in any of the 3 transmembrane regions described previously) versus nontransmembrane or nonmissense (i.e., any other identified LQT1 to LQT3 mutation that was not transmembrane-missense).
Statistical analysis
The clinical characteristics of study patients were compared by genotype and QTc categories using chi-square tests for categorical variables and t tests and Mann-Whitney-Wilcoxon tests for continuous variables. The Kaplan-Meier estimator was used to assess the time to a first life-threatening event and the cumulative event rates by risk groups and risk factors, and groups were compared using the log-rank test.
Cox proportional hazards regression analysis was carried out in the total study population and separately in the subset of patients with genotype-positive LQTS. Pre-specified covariates in the total population model included the 3 genotype and QTc categories, sex, and time-dependent beta-blocker therapy. The models comprising genotype-positive patients included the following pre-specified covariates: QTc category (normal range [≤440 ms] vs. prolonged [>440 ms]), the LQT1 to LQT3 genotypes, mutation location and type, sex, QTc duration (assessed both as a continuous measure [per 10-ms increase] and as a categorical covariate [dichotomized at the median value of each QTc category and assessed in separate models]), time-dependent beta-blocker therapy, and a family history of SCD in a first-degree relative. The effect of each covariate on outcome in each QTc category (i.e., in patients with LQTS with normal-range and prolonged QTc intervals) was assessed using interaction-term analysis, with interactions tested 1 at a time. Estimates of predictor hazard ratios in the separate normal and prolonged QTc categories were obtained using these interactions. To avoid violation of the proportional hazards assumption due to sex-risk crossover during adolescence, we used an age-sex interaction term in the multivariate models.
Because almost all the subjects were first-degree and second-degree relatives of probands, the effect of lack of independence between subjects was evaluated in the Cox model with grouped jackknife estimates for family membership (14). All grouped jackknife standard errors for the covariate risk factors fell within 3% of those obtained from the unadjusted Cox model, and therefore only the Cox model findings are reported. The statistical software used for the analyses was SAS version 9.20 (SAS Institute Inc., Cary, North Carolina). A 2-sided significance level of 0.05 was used for hypothesis testing.
Results
The spectrum and number of LQT1-associated, LQT2-associated, and LQT3-associated mutations by the pre-specified location and type categories are presented in Online Table 1. Totals of 100, 177, and 41 different mutations were identified in the KCNQ1-encoded Kv7.1, KCNH2-encoded Kv11.1, and SCN5A-encoded Nav1.5 ion channels, respectively. Study patients with identified LQTS mutations exhibited a very wide QTc interval distribution (Fig. 1), ranging from a minimum of 350 ms to a maximum of 800 ms (mean 450 ± 56 ms; median 440 ms; interquartile range: 410 to 480 ms). QTc distribution was similar among the 3 LQTS genotypes. Four hundred sixty-nine LQTS mutation–positive patients exhibited normal-range QTc intervals, constituting 25% of identified cases.
Table 1.
Baseline and Follow-Up Characteristics of the Study Population by Genotype-Phenotype
| Characteristic | Unaffected Family Members (n = 1,525) | Patients With LQTS With Normal-Range QTc Intervals (n = 469) | Patients With LQTS With Prolonged QTc Intervals (n = 1,392) |
|---|---|---|---|
| Female | 52% | 48% | 61%*† |
| Family history of SCD | 8% | 12% | 19%*† |
| QTc interval (ms) | |||
| Mean ± SD | 412 ± 22 | 419 ± 20 | 501 ± 48 |
| Median (IQR) | 420 (400–430) | 420 (410–440) | 490 (470–520) |
| Proband | 8% | 8% | 29%*† |
| RR interval (ms) | |||
| Mean ±SD | 793 ± 221 | 888 ± 236 | 848 ± 214*† |
| Median (IQR) | 800 (640–930) | 900 (740–1,040) | 840 (700–1,000)*† |
| Genotype | |||
| LQT1 | NA | 40% | 39% |
| LQT2 | NA | 45% | 47% |
| LQT3 | NA | 16% | 14% |
| Mutation: TM-MS | |||
| Overall | NA | 35% | 43% |
| LQT1 | NA | 45% | 61% |
| LQT2 | NA | 16% | 29%† |
| LQT3 | NA | 64% | 31%† |
| Therapies | |||
| Beta-blockers | 6.2% | 38% | 54%*† |
| Pacemaker | 0.3% | 0.6% | 5%*† |
| LCSD | 0.1% | 0.2% | 1.4%*† |
| ICD | 0.6% | 6% | 14%*† |
| Events | |||
| Syncope | 10% | 21% | 40%*† |
| ACA | 0.2% | 1.3% | 8.4%*† |
| SCD | 0.1% | 1.5% | 4.4%*† |
| ACA/SCDठ| 0.3% | 2.8% | 11.3%* |
p < 0.05 for the comparison among the 3 genotyped categories.
p < 0.05 for the comparison between genotype-positive patients with QTc intervals ≤440 ms and genotype-positive patients with QTc intervals >440 ms.
Appropriate ICD shocks constituted 0.04% of ACAs in genotype-positive patients with QTc intervals ≤440 ms and 1.4% of ACAs in genotype-positive patients with QTc intervals >440 ms.
Only the first event for each patient was considered.
ACA = aborted cardiac arrest; ICD = implantable cardioverter-defibrillator; IQR = interquartile range; LCSD = left cardiac sympathetic denervation; LQT1 = long-QT syndrome type 1; LQT1 = long-QT syndrome type 2; LQT3 = long-QT syndrome type 3; LQTS = long-QT syndrome; MS = missense; NA = not applicable; QTc = corrected QT; SCD = sudden cardiac death; TM = transmembrane.
Figure 1. Distribution of QTc Interval Duration in Genotype-Positive Patients With LQTS.
Distribution of corrected QT (QTc) interval durations in genotype-positive study patients. LQTS = long-QT syndrome.
The clinical characteristics of the total study population by genotype and QTc subgroup are shown in Table 1. The frequency of probands (defined in the registry as the first person in a family, living or deceased, identified to have LQTS by the enrollment center) was highest in patients with prolonged QTc intervals, whereas most patients with normal-range QTc intervals (92%) were asymptomatic at the time of genetic testing. The frequency of female subjects was similar between the unaffected subjects and patients with LQTS with normal-range QTc intervals and higher in patients with prolonged QTc intervals. In mutation carriers, the frequency of the 3 main LQTS genotypes was similar between patients with and without prolonged QTc intervals. However, patients with LQT1 and LQT2 with prolonged QTc intervals had a higher frequency of transmembrane-missense mutations compared with the corresponding genotype carriers who had normal-range QTc intervals. LQTS-related therapies were administered to a significantly higher frequency of patients with prolonged QTc intervals than to subjects in the other 2 subgroups (Table 1).
Clinical course by genotype and QTc subgroup
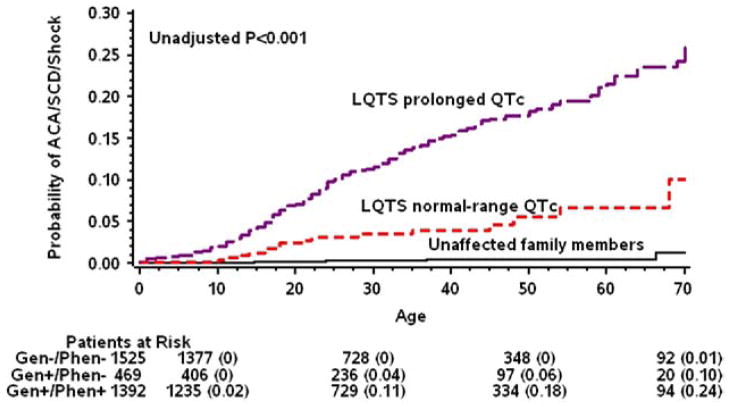
Kaplan-Meier survival analysis (Fig. 2) demonstrated a relatively low rate of ACA or SCD in patients with LQTS with normal-range QTc intervals (4% at age 40 years and 10% at age 70 years). Event rates were significantly higher in patients with prolonged QTc intervals (15% and 24% at age 70 years; log-rank p < 0.001 for the comparison with the normal-range QTc subgroup) and significantly lower in unaffected family members (0.4% and 1% at age 70 years; log-rank p < 0.001 for the comparison with the normal-range QTc subgroup and for the overall difference among the 3 subgroups). Notably, life-threatening events in patients with normal-range QTc intervals occurred mostly after age 10 years, whereas patients with prolonged QTc intervals exhibited an earlier onset of life-threatening events (Fig. 2).
Figure 2. Rate of ACA or SCD by Genotype and QTc Category.
Kaplan-Meier cumulative probabilities of aborted cardiac arrest (ACA) and sudden cardiac death (SCD) by genotype and corrected QT (QTc) subgroup. LQTS = long-QT syndrome.
After multivariate adjustment for sex, time-dependent beta-blocker therapy, and a family history of SCD in a first-degree relative, patients with LQTS with normal-range QTc intervals were shown to have a significant 72% (p < 0.001) lower risk for ACA or SCD compared with patients with prolonged QTc intervals but also exhibited a >10-fold increase in the risk for life-threatening events compared with unaffected family members (Table 2). Histories of syncope were present in 62% of patients with LQTS with normal-range QTc intervals who had life-threatening events during follow-up. Accordingly, when the composite secondary end point of a first cardiac event of any type was assessed (comprising mainly non-life-threatening syncopal episodes), patients with normal-range QTc intervals were consistently shown to be at a lower risk compared with those with prolonged QTc intervals (hazard ratio [HR]: 0.47; 95% confidence interval [CI]: 0.33 to 0.59; p < 0.001) and at a higher risk compared with unaffected family members (HR: 5.20; 95% CI: 4.19 to 6.44; p < 0.001).
Table 2.
Multivariate Analysis: Risk for ACA or SCD Among the 3 Genotype and QTc Categories*
| Genotype and QTc Subgroup | HR | 95% CI | p Value |
|---|---|---|---|
| LQTS with prolonged QTc interval vs. unaffected family members | 36.53 | 13.35–99.95 | <0.001 |
| LQTS with normal-range QTc interval vs. unaffected family members | 10.25 | 3.34–31.46 | <0.001 |
| LQTS with normal-range QTc interval vs. LQTS with prolonged QTc interval | 0.28 | 0.16–0.49 | <0.001 |
Model also adjusted for sex (female age >13 years) and time-dependent beta-blocker therapy.
CI = confidence interval; HR = hazard ratio; other abbreviations as in Table 1.
Risk factors for ACA or SCD in patients with LQTS with and without prolonged QTc intervals
Interaction-term analysis demonstrated significant differences in risk factors for life-threatening events between the 2 LQTS subgroups (Table 3). In patients with normal-range QTc intervals, the LQT1 and LQT3 genotypes were associated with respective 10- and 8-fold increases in the risk for life-threatening events compared with the LQT2 genotype. In contrast, in patients with prolonged QTc intervals, the LQT1 genotype was associated with one-half the risk of the LQT2 genotype (p = 0.002), with a statistically significant genotype– by–QTc subgroup interaction (p = 0.006) (Table 3, first row), and the LQT3 genotype showed a similar risk to the LQT2 genotype, without a statistically significant genotype–by–QTc subgroup interaction (Table 3, second row).
Table 3.
Risk Factors for ACA or SCD in Patients With LQTS by QTc Interval Category*
| Variable | LQTS and Normal-Range QTc Interval
|
LQTS and Prolonged QTc Interval
|
p Value for Interaction | ||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Genotype | |||||
| LQT1 vs. LQT2 | 9.88 (1.26–37.63) | 0.03 | 0.53 (0.35–0.79) | 0.002 | 0.006 |
| LQT3 vs. LQT2 | 8.04 (0.85–36.03) | 0.07 | 1.07 (0.70–1.63) | 0.77 | 0.08 |
| Mutation location and type | |||||
| TM-MS vs. non-TM-MS | 6.32 (1.71–23.33) | 0.006 | 1.24 (0.88–1.76) | 0.22 | 0.02 |
| Sex | |||||
| Female age >13 yrs vs. male age >13 yrs | 1.32 (0.42–4.17) | 0.64 | 1.90 (1.26–2.86) | 0.002 | 0.53 |
| QTc interval (ms) | |||||
| Per 10-ms increase | 1.20 (0.81–1.78) | 0.35 | 1.08 (1.05–1.10) | <0.001 | 0.58 |
| ≥Median vs. < median† | 1.03 (0.36–2.98) | 0.95 | 2.96 (2.06–4.26) | <0.001 | NA |
Cox proportional hazards regression modeling was carried out in models that included all patients with genotype-positive LQTS (n = 1,861). Covariates in the models included QTc category (≤440 ms vs. >440 ms), genotype, mutation location and type, sex, QTc interval (assessed as a continuous measure [per 10-ms increase]), time-dependent beta-blocker therapy, and a family history of SCD; the effect of each covariate in patients with normal-range (≤440 ms) and those with prolonged (>440 ms) QTc intervals was assessed by interaction-term analysis, with interactions tested 1 at a time. Estimates of predictor hazard ratios in the separate normal-range and prolonged QTc interval groups were obtained using these interactions. Virtually identical results for all pre-specified risk factors were also obtained from the models that did not include appropriate ICD shocks as part of the composite end point.
Results were obtained from separate models that assessed the risk associated with QTc values greater than or equal to the median in patients with LQTS with normal-range QTc intervals (median 420 ms) and prolonged QTc intervals (median 500 ms).
The location and type of the LQTS mutation were shown to be significant risk factors for ACA or SCD in patients with normal-range QTc intervals. In this LQTS subset, transmembrane-missense mutations were associated with a pronounced >6-fold (p = 0.006) increase in the risk for ACA or SCD compared with nontransmembrane or nonmissense mutations. In contrast, in patients with prolonged QTc intervals, transmembrane-missense mutations were not independently associated with outcomes (Table 3, third row). Notably, when the secondary end point of cardiac events of any type was assessed, transmembrane-missense mutations were shown to be an independent risk factor in both LQTS subgroups (normal-range QTc interval, HR: 1.71; 95% CI: 1.16 to 2.34; prolonged QTc interval, HR: 1.39; 95% CI: 1.17 to 1.65).
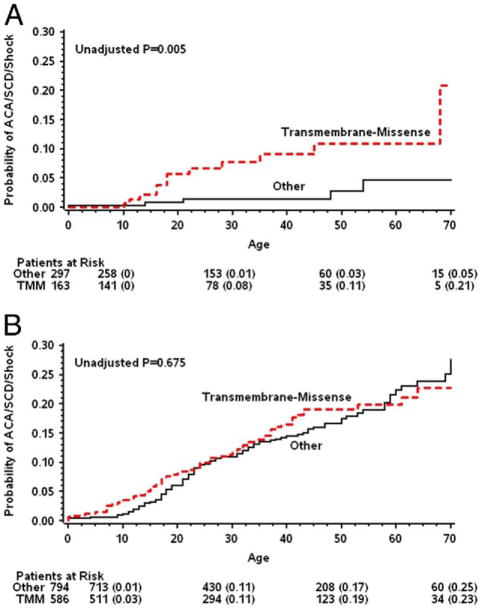
Consistent results demonstrating an association between transmembrane-missense mutations and the risk for ACA or SCD in patients with normal-range QTc intervals were shown when the reference group (comprising nontrans-membrane or nonmissense mutations) was further divided into 3 subcategories, including nonmissense mutations in the transmembrane region, missense mutations in the non-transmembrane region, and nonmissense mutations in the nontransmembrane region (HR >4.0 for all 3 comparisons). Accordingly, patients with normal-range QTc intervals with transmembrane-missense mutations experienced a relatively high rate of ACA or SCD during follow-up (9% at age 40 years and 21% at age 70 years), whereas patients with normal-range QTc intervals with other mutations had a very low event rate (1% at age 40 years and 5% at age 70 years; log-rank p for overall difference = 0.005) (Fig. 3A). In contrast, in patients with prolonged QTc intervals, there was no statistically significant difference in the rate of ACA or SCD between the 2 mutation categories (16% and 14% at 40 years, respectively, p = 0.18) (Fig. 3B).
Figure 3. Rate of ACA or SCD in Patients With Normal-Range and Prolonged QTc Intervals by Mutation Location and Type.
Kaplan-Meier cumulative probabilities of aborted cardiac arrest (ACA) and sudden cardiac death (SCD) by mutation location and type in patients with long-QT syndrome (LQTS) with (A) corrected QT (QTc) intervals ≤440 ms and (B) QTc intervals >440 ms.
Clinical and ECG factors, including sex and QTc duration, were shown to be associated with a significant increase in the risk for ACA or SCD only in patients with prolonged QTc intervals (Table 3, rows 4 to 6). In contrast, in patients with normal-range QTc intervals, sex was not a significant risk factor, and QTc duration was not independently associated with a significant increase in the risk for ACA or SCD when assessed as a continuous measure or when dichotomized at the median value (≥420 ms).
As suggested previously (15), the presence of a family history of SCD in any first-degree relative was not shown to be an independent predictor of ACA or SCD in patients with either normal-range QTc intervals (HR: 0.89; 95% CI: 0.63 to 1.25; p = 0.50) or prolonged QTc intervals (HR: 1.40; 95% CI: 0.32 to 6.17; p = 0.65) after adjustment for genetic and clinical factors.
Beta-blocker therapy was administered to 38% of patients who had normal-range QTc intervals compared with 54% of the patients who had prolonged QTc intervals (p < 0.001) (Table 1). Treatment with beta-blockers was associated with an overall significant 25% reduction in the risk for ACA or SCD in the total study population (95% CI: 0.70 to 0.80; p < 0.001), with similar effects in patients with normal-range QTc intervals and those with prolonged QTc intervals (p for beta-blocker–by–LQTS subset interaction = 0.45).
Characteristics of fatal or near-fatal cases with a normal-range QTc intervals
The characteristics of patients with normal-range QTc intervals who experienced ACA or SCD during follow-up are shown in Table 4. The mean age at occurrence of the lethal or near-lethal event in this population was 25.9 ± 4.5 years. Nine of the patients (53%) who experienced events were women, and 4 (24%) were treated with beta-blockers are the time of the events. In patients with normal-range QTc intervals with available data regarding therapies and triggers at the time of the events, none were reported as being treated with a QT interval–prolonging drugs at the time of ACA or SCD, and the majority of the lethal or near-lethal events were not associated with exercise or arousal triggers (Table 4).
Table 4.
Characteristics of ACA and SCD Cases With Normal-Range QTc Intervals
| Case | Event | Event Age (yrs) | Female | QTc Interval (ms) | BB† | LCSD‡ | PM‡ | ICD‡ | QT PD | Trigger* | Genotype | Mutation Location and Type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SCD | 0.5 | − | 390 | − | − | − | − | − | NA | LQT3 | Non-TM-MS |
| 2 | ACA | 10 | 430 | − | − | − | − | − | Exercise | LQT1 | TM-MS | |
| 3 | ACA/shock | 11 | + | 400 | − | − | − | + | − | Non-E/A | LQT1 | TM-MS |
| 4 | SCD | 13 | − | 440 | + | − | − | − | NA | NA | LQT1 | TM-MS |
| 5 | ACA | 14 | − | 410 | − | − | − | − | − | Exercise | LQT1 | Non-TM-MS |
| 6 | SCD | 16 | + | 420 | − | − | − | − | − | Non-E/A | LQT3 | TM-MS |
| 7 | ACA | 16 | + | 440 | + | − | − | − | − | Arousal | LQT1 | TM-MS |
| 8 | SCD | 18 | − | 430 | + | − | − | − | − | Non-E/A | LQT1 | TM-MS |
| 9 | ACA | 18 | + | 410 | − | − | − | − | − | Exercise | LQT1 | TM-MS |
| 10 | SCD | 21 | + | 380 | − | − | − | − | − | Arousal | LQT2 | Non-TM-MS |
| 11 | SCD | 22 | − | 440 | − | − | − | − | NA | NA | LQT1 | TM-MS |
| 12 | SCD | 28 | − | 410 | − | − | − | − | − | Exercise | LQT1 | TM-MS |
| 13 | ACA | 35 | + | 420 | − | − | − | − | − | Non-E/A | LQT3 | TM-MS |
| 14 | ACA | 46 | + | 440 | + | − | − | − | NA | NA | LQT2 | TM-MS |
| 15 | SCD | 48 | − | 430 | + | − | − | − | − | Non-E/A | LQT2 | Non-TM-MS |
| 16 | ACA | 54 | + | 420 | − | − | − | − | − | Non-E/A | LQT3 | Non-TM-MS |
| 17 | SCD | 69 | − | 380 | − | − | − | − | NA | NA | LQT1 | TM-MS |
Data regarding triggers for cardiac events and treatment with QT interval–prolonging medications were available for study patients who were enrolled in the U.S. portion of the International LQTS Registry.
At time of event.
Implanted or performed before event.
Discussion
In this study, we assessed the clinical courses and risk factors for life-threatening events in LQTS patients with genetically-confirmed LQTS who do not exhibit the disease’s phenotypic hallmark of QT interval prolongation, otherwise referred to as concealed LQTS, normal–QT interval LQTS, or genotype-positive/ECG phenotype–negative LQTS. Similar to prior studies (16), we have shown that patients with LQT1 to LQT3 exhibit a wide QTc distribution, with approximately 25% having QTc intervals well within the normal range. The rate of ACA or SCD in patients with LQTS with normal-range QTc intervals was shown to be very low (4% from birth through age 40 years, corresponding to an approximate event rate of 0.13% per year). Comparatively, however, this very low risk subset of the LQTS population still exhibited a >10-fold increase in the risk for life-threatening events compared with genetically and phenotypically unaffected family members. Importantly, predictors of life-threatening events were shown to be significantly different between LQTS patients with and without prolonged QTc intervals. In the latter LQTS subgroup, genetic data, including knowledge of genotype and mutation characteristics, were shown to identify the risk for ACA or SCD, whereas in the former LQTS subgroup, female sex in the post-adolescence period and QTc duration were identified as the predominant risk factors for life-threatening events.
The clinical courses of patients with LQTS are variable because of incomplete penetrance (17). They are influenced by age, genotype, sex, environmental factors, therapy, and possibly other modifier genes (1–10). Recent studies from the International LQTS Registry that assessed the risk for life-threatening events in patients with LQTS have consistently demonstrated that ECG and clinical risk factors, including the QTc interval and age-sex interactions, identify increased risk in the LQTS population (3–5). These studies, however, included mainly phenotype-positive patients with LQTS with QTc intervals ≥450 ms. Thus, the effect of genetic data on outcomes in these studies was not statistically significant after adjustment for the ECG and clinical factors. The present study population, comprising 1,861 genetically confirmed patients with the LQT1 to LQT3 genotypes, extends the data derived from prior studies and demonstrates that risk factors for life-threatening events are significantly different between patients with LQTS with and without QTc prolongation. Consistent with prior studies, we have shown that in patients with LQTS who exhibit prolonged QTc durations, ECG information and clinical factors can be used to identify the risk for life-threatening events. In contrast, in mutation-positive subjects with normal-range QTc intervals, genetic factors, including knowledge of the LQTS genotypes and the mutation location and type, identified patients who were at an increased risk for ACA or SCD after adjustment for ECG and clinical data.
Sex was not a significant risk factor for cardiac events in patients with normal-range QTc intervals. Furthermore, patients with normal-range QTc intervals displayed a similar frequency of women as unaffected family members, whereas the frequency of women was signifi-cantly higher among patients with prolonged QTc intervals. These findings are in accordance with earlier evidence of longer QTc intervals in LQTS women than in men (18), resulting in a marked female predominance in phenotypically affected patients (3–5). The biologic basis for this sex difference might be the down-regulation of expression of cardiac potassium-channel genes by female sex hormones, which have been shown to prolong the QT interval in both congenital and drug-induced LQTS (19,20). These hormonal effects may explain the present findings of a lower frequency of LQTS women with normal-range QTc intervals.
Recent genotype-phenotype studies have shown that missense mutations located in the transmembrane region, which is responsible for forming the ion conduction pathway of the channel, are associated with a significantly higher risk for cardiac events compared with mutations that are located in other regions of the LQTS channel (9,10). The present study also shows that transmembrane-missense mutations are associated with a significantly higher risk for cardiac events of any type (predominated by syncopal episodes) in patients with LQTS with both normal-range and prolonged QTc intervals. However, our findings suggest that data regarding mutation characteristics are important for the assessment of life-threatening events (comprising ACA and SCD) mainly in patients with normal-range QTc intervals, in whom information derived from ECG and clinical data is more limited. In this LQTS subset, missense mutations located in the trans-membrane region were shown to be associated with a >6-fold increase in the risk for life-threatening events and with a clinically meaningful rate of ACA or SCD (9%) from birth through age 40 years.
The mechanisms relating to the occurrence of life-threatening ventricular tachyarrhythmias in phenotype-negative patients with LQTS are not clear. In the present study, none of the patients with normal-range QTc intervals who experienced ACA or SCD took QT interval–prolonging medications at the time of the events. Furthermore, most events in patients with normal-range QTc intervals were not related to exercise or arousal triggers (Table 4). An ECG tracing from a patient with the LQT1 genotype who developed arrhythmic events despite a normal-range QTc interval showed spontaneous generation of polymorphic ventricular tachycardia without preceding extrasystolic pauses or sudden sinus rate acceleration (Fig. 4), possibly explaining the occurrence of ACA or SCD in study patients with normal-range QTc intervals who were treated with beta-blockers at the time of the events.
Figure 4. Polymorphic Ventricular Tachycardia in a Patient With a Normal-Range QTc Interval.
Spontaneous generation of polymorphic ventricular tachycardia in a patient with long-QT syndrome type 1 with a normal-range corrected QT (QTc) interval. (A) The patient had a QTc duration of 410 ms on baseline electrocardiography. (B) Electrocardiographic tracing at the time of arrhythmic event demonstrates sinus rate with an RR interval of 1,000 ms without significant QT prolongation before the arrhythmia. (C) The patient was treated with nadolol and received an implantable cardioverter-defibrillator but continued to exhibit arrhythmic episodes that were recorded on implantable cardioverter-defibrillator interrogation.
Study limitations
Most study patients did not undergo comprehensive genetic testing for all currently known mutations that may predispose to arrhythmic risk. Thus, it is possible that the coexistence of modifier genes affected the outcomes of patients with LQTS with normal-range QTc intervals who experienced life-threatening cardiac events. In addition, to provide an estimation of event rates among unaffected family members, we included in the control group subjects who were both genotype negative and also had normal-range QTc intervals (and excluded genotype-negative subjects with prolonged QTc intervals due to possible unidentified mutations in this subset). Therefore, the overall frequency of genotype-positive subjects in the total population may not represent the true penetrance of LQTS in affected families.
The threshold value of 440 ms for the definition of a normal-range QTc in the present study was based on the diagnostic criteria for LQTS proposed by Schwartz et al. (12), which define a prolonged QTc interval as ≥450 ms in male patients and ≥460 ms in female patients. We chose to use a uniform approach by selecting 440 ms as the upper limit of normal rather than having separate phenotypic definitions for male and female patients. It should also be noted that 2.5% of infants and 10% to 20% of adults exceed this cutoff (21). Thus, the 440-ms value is not meant to suggest an LQTS diagnosis on its own.
Conclusions
The present study shows that patients with LQTS who exhibit normal-range QTc intervals constitute approximately 25% of the LQTS population and have a signifi-cantly lower risk for life-threatening events compared with phenotypically affected patients but also exhibit a significant increase in the risk of ACA or SCD compared with unaffected family members. Missense mutations in the transmembrane regions of the ion channels, mainly in patients with LQT1 and LQT3, were shown to identify patients with normal-range QTc intervals who have an increased risk for ACA or SCD. In contrast, increments in QTc duration were not shown to be significantly associated with increased risk for life-threatening events in this population. These findings suggest that: 1) risk assessment in phenotype-negative family members of LQTS probands should include genetic testing, because a positive genetic test result in a family member with a normal-range QTc interval implies an overall >10-fold increase in the risk for ACA or SCD compared with a negative test result in an unaffected family member; 2) genetic data may be used to identify phenotype-negative patients with LQTS who are at increased risk for fatal ventricular tachyarrhythmias independently of QTc duration; and 3) LQTS mutation–positive patients with normal-range QTc intervals who are identified as having increased risk for life-threatening events on the basis of genotype and mutation characteristics (i.e., LQT1 and LQT3 with transmembrane-missense mutations) should be carefully followed and receive a similar management strategy as phenotype-positive patients with LQTS, including avoidance of QT-prolonging medications (22), routine therapy with beta-blockers, and possibly implantable cardioverter-defibrillator therapy in those who remain symptomatic despite medical therapy. Conversely, patients with the lowest risk profile of already low risk, concealed LQTS (i.e., concealed LQT2 and non-transmembrane-missense LQT1 and LQT3) may represent the nominally near zero risk subpopulation(s) of LQTS in need of only preventative health recommendations such as QT drug avoidance.
Acknowledgments
This work was supported by research grants HL-33843 and HL-51618 from the National Institutes of Health.
Abbreviations and Acronyms
- ACA
aborted cardiac arrest
- ECG
electrocardiographic
- LQTS
long-QT syndrome
- LQT1
long-QT syndrome type 1
- LQT2
long-QT syndrome type 2
- LQT3
long-QT syndrome type 3
- QTc
corrected QT interval
- SCD
sudden cardiac death
APPENDIX
For a table about KCNQ1, KCNH2, and SCN5A mutations by amino acid coding, frequency, location, and type, please see the online version of this article.
Footnotes
The authors have reported that they have no relationships to disclose.
References
- 1.Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–44. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51:2291–300. doi: 10.1016/j.jacc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg I, Moss AJ, Peterson DR, et al. Risk factors for aborted cardiac arrest and sudden cardiac death in children with the congenital long-QT syndrome. Circulation. 2008;29(117):2184–91. doi: 10.1161/CIRCULATIONAHA.107.701243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobbs JB, Peterson DR, Moss AJ, et al. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA. 2006;296:1249–54. doi: 10.1001/jama.296.10.1249. [DOI] [PubMed] [Google Scholar]
- 5.Sauer AJ, Moss AJ, McNitt S, et al. Long QT syndrome in adults. J Am Coll Cardiol. 2007;49:329–37. doi: 10.1016/j.jacc.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 6.Zareba W, Moss AJ, Locati EH, et al. International Long QT Syndrome Registry. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol. 2003;42:103–9. doi: 10.1016/s0735-1097(03)00554-0. [DOI] [PubMed] [Google Scholar]
- 7.Zareba W, Moss AJ, Schwartz PJ, et al. International Long-QT Syndrome Registry Research Group. Influence of genotype on the clinical course of the long-QT syndrome. N Engl J Med. 1998;339:960–5. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 8.Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–74. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 9.Moss AJ, Shimizu W, Wilde AA, et al. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115:2481–9. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu W, Moss AJ, Wilde AA, et al. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol. 2009;54:2052–62. doi: 10.1016/j.jacc.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazett HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–67. [Google Scholar]
- 12.Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome: an update. Circulation. 1993;88:782–4. doi: 10.1161/01.cir.88.2.782. [DOI] [PubMed] [Google Scholar]
- 13.Moss AJ, Kass RS. Long QT syndrome: from channels to cardiac arrhythmias. J Clin Invest. 2005;115:2018–24. doi: 10.1172/JCI25537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer-Verlag; 2000. [Google Scholar]
- 15.Kaufman ES, McNitt S, Moss AJ, et al. Risk of death in the long QT syndrome when a sibling has died. Heart Rhythm. 2008;5:831–6. doi: 10.1016/j.hrthm.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med. 1992;327:846–52. doi: 10.1056/NEJM199209173271204. [DOI] [PubMed] [Google Scholar]
- 17.Priori SG, Napolitano C, Schwartz PJ. Low penetrance in the long-QT syndrome: clinical impact. Circulation. 1999;99:529–33. doi: 10.1161/01.cir.99.4.529. [DOI] [PubMed] [Google Scholar]
- 18.Stramba-Badiale M, Locati EH, Martinelli A, Courville J, Schwartz PJ. Gender and the relationship between ventricular repolarization and cardiac cycle length during 24-h Holter recordings. Eur Heart J. 1997;18:1000–6. doi: 10.1093/oxfordjournals.eurheartj.a015357. [DOI] [PubMed] [Google Scholar]
- 19.Malloy KJ, Bahinski A. Cardiovascular disease and arrhythmias: unique risks in women. J Gend Specif Med. 1999;2:37–44. [PubMed] [Google Scholar]
- 20.Lehmann MH, Hardy S, Archibald D, Quart B, MacNeil DJ. Sex difference in risk of torsade de pointes with d,l-sotalol. Circulation. 1996;94:2535–41. doi: 10.1161/01.cir.94.10.2535. [DOI] [PubMed] [Google Scholar]
- 21.Johnson JN, Ackerman MJ. QTc: how long is too long? Br J Sports Med. 2009;3:657–62. doi: 10.1136/bjsm.2008.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent GM, Schwartz PJ, Denjoy I, et al. High efficacy of β-blockers in long-QT syndrome type 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of β-blocker treatment “failures”. Circulation. 2009;20(119):215–21. doi: 10.1161/CIRCULATIONAHA.108.772533. [DOI] [PubMed] [Google Scholar]