Abstract
We describe the case of a newborn with congenital long QT syndrome, with 2:1 AV block and frequent episodes of Torsades de Pointes (TdP) requiring placement of a dual chamber ICD at 33 days and 3.63 kg, the youngest and smallest patient, thus far reported. Long QT syndrome was diagnosed due to bradycardia in the newborn nursery, with frequent episodes of TdP. The patient was initially treated with magnesium and esmolol then given lidocaine which resulted in dramatic transient normalization of the QTc with 1:1 AV nodal conduction. An attempt to transition to oral sodium channel and beta blockade was unsuccessful. An ICD was placed and dual chamber pacing was initiated which facilitated the transition to an oral medical regimen and ultimate discharge from the hospital. Soon after placement of the ICD, genetic testing revealed a novel F1473C mutation in the SCN5A gene. Episodes of TdP continued and left stellate gangliectomy was performed at 3 months of age. At 30 months follow-up, the patient has occasional, self-limited episodes of TdP and has received rare, successful, and appropriate ICD shocks.
Keywords: Pediatric, Long QT Syndrome, SCN5A, Implantable cardioverter defibrillator, Sympathectomy
1 Case report
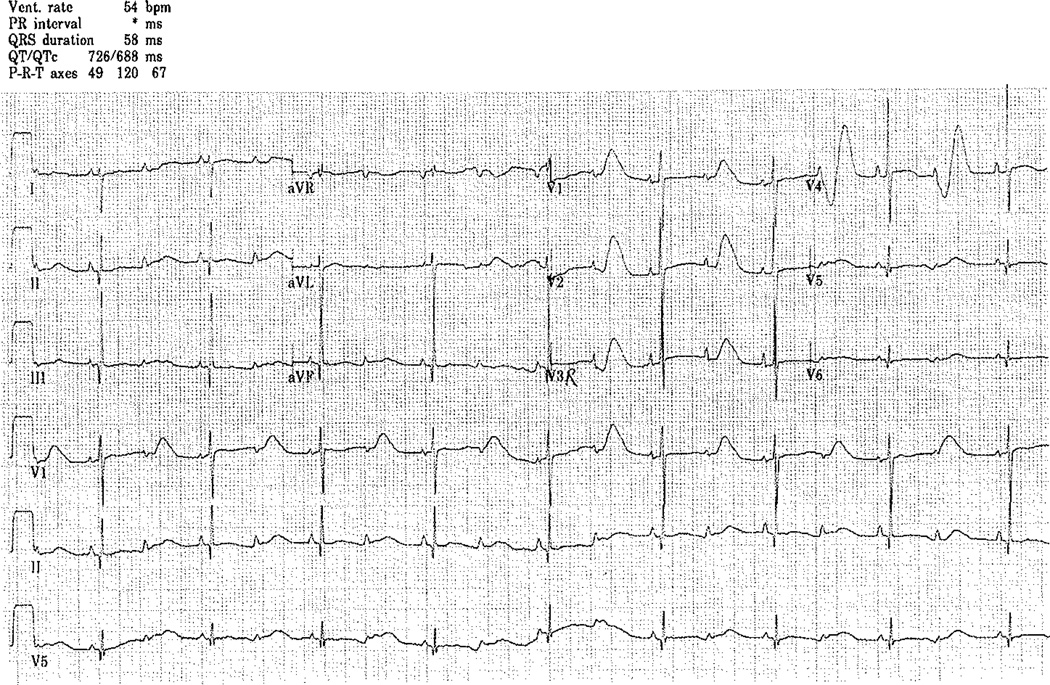
A full-term infant boy was born via normal vaginal delivery to a first-time mother. He was evaluated prenatally for bradycardia. Fetal echocardiogram was normal. There was no family history of arrhythmias, sudden death, or deafness. At birth, he weighed 3.23 kg and was bradycardic with a heart rate varying between 60 and 100 bpm. His physical exam was normal. An ECG demonstrated 2:1 AV block with a corrected QT interval of 790 ms with multiple episodes of TdP (Fig. 1).
Fig. 1.
Initial ECG revealing bradycardia with 2:1 AV block secondary to a long QT interval
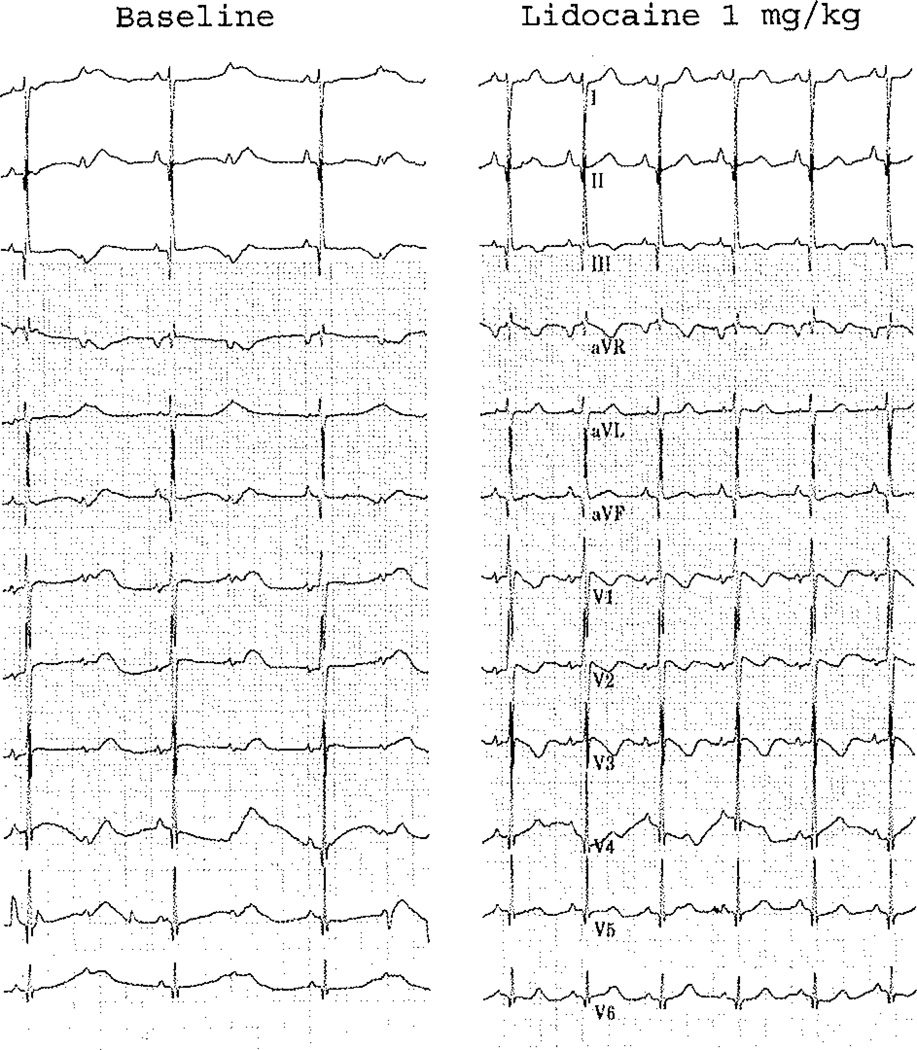
He was treated with intravenous magnesium, calcium, and an esmolol infusion (100 µg/kg/min). He was transferred to our institution where he continued to experience short episodes of TdP. Due to the T wave appearance on his ECG, the diagnosis of a sodium channel defect was considered, and the patient was given a dose of intravenous lidocaine, which resulted in a dramatic shortening of the QT interval. Figure 2 demonstrates selected beats from a of lidocaine, with normalization of his QTc and conversion to 1:1 AV nodal conduction. In light of this dramatic response, the suspicion of a SCN5A defect was strengthened, and a lidocaine infusion was initiated (20 µg/kg/min). QT interval remained under 600 ms with 1:1 conduction. Of note, a hearing screen was normal, and there was no family history of deafness.
Fig. 2.
Selected beats from a rhythm strip over a 15-s interval during which a lidocaine bolus was administered demonstrating a dramatic shortening of the QT interval
Prior to transitioning to oral medical therapy, he developed necrotizing enterocolitis (NEC), which was managed medically over a 2-week period. One week into therapy for NEC, his antibiotic regimen was changed from ampicillin and gentamycin to vancomycin and piperacillin-tazobactam, with subsequent prolongation of the QTc (640 ms) and return of 2:1 AV block. The vancomycin and piperacillin-tazobactam were stopped, and within 12 h, the QTc shortened (540 m) and he again developed 1:1 AV nodal conduction.
He was ultimately transitioned to oral beta blocker (propranolol) and sodium channel blockade with both flecainide and mexiletine. Despite 175 mg/m2/day of flecainide, he again developed significant lengthening of his corrected QTc resulting in 2:1 AV nodal conduction with multiple episodes of TdP. Intravenous lidocaine and esmolol were resumed, and at 33 days of life, weighing 3.63 kg, an epicardial ICD was implanted.
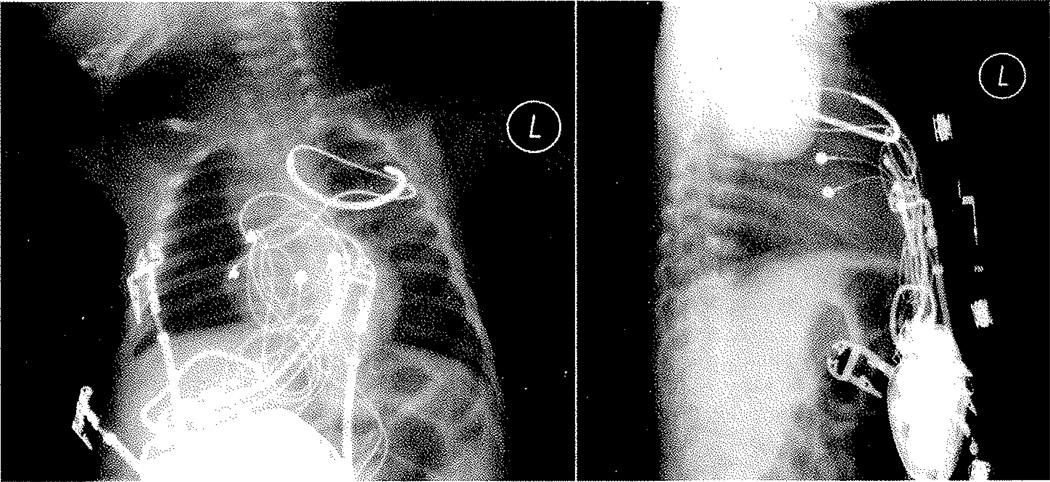
The ICD was placed via a median sternotomy. A bipolar epicardial lead (Bipolar CapsureEpi IS-1, Model 4968, Medtronic, Minneapolis, MN, USA) was placed on the surface of the right atrium and two unipolar leads (Unipolar CapsureEpi Bipolar IS-1, Model 4965, Medtronic) were connected using an adapter (Spectrax VL VVIOO 4.75 Bif/Bi, Model 5966, Medtronic) approximating a bipolar lead on the surface of the right ventricle. In an attempt to maximize ventricular sensing, the leads were placed far apart on the ventricular epicardium, one on the RV apex and the other on the right ventricular outflow tract. Testing revealed a sensed amplitude of 25 mV and adequate pacing thresholds (<1.5 V). A shocking coil was placed into the left pleural space posterior to the heart in the second intercostal space (Trasvene DF-1 SVC, Model 6937, Medtronic). A pocket was then fashioned in the preperitoneal space via blunt dissection in order to house the ICD (Vitality 2 DR IS-l/DF-1, Model T165, Boston Scientific, St. Paul, MN, USA), which was placed without difficulty (Fig. 3).
Fig. 3.
Chest radiograph, PA, and lateral demonstrating ICD position, with atrial and ventricular leads and shocking coil located in the left pleural space
Vigorous attempts were made to induce ventricular fibrillation for defibrillation testing. Despite cessation of lidocaine and esmolol (each for five half-lives), attempts at induction at baseline and after initiation of isoproterenol and epinephnne using shock on T wave programming and repeated 50-Hz bursts were unsuccessful. A dose of lidocaine was administered in an attempt to shorten the QTc and facilitate TdP, but this was likewise unsuccessful. In light of a lack of a demonstration of efficacy, the device was empirically programmed to defibrillate with 31 J for ventricular rates greater than 205 bpm. DDD pacing was initiated at a lower rate of 100 bpm with a very short AV delay (40 ms) in an attempt to shorten the QTc with ventricular pacing, and he was restarted on lidocaine (25 µg/kg/min) and esmolol (150 µg/kg/min).
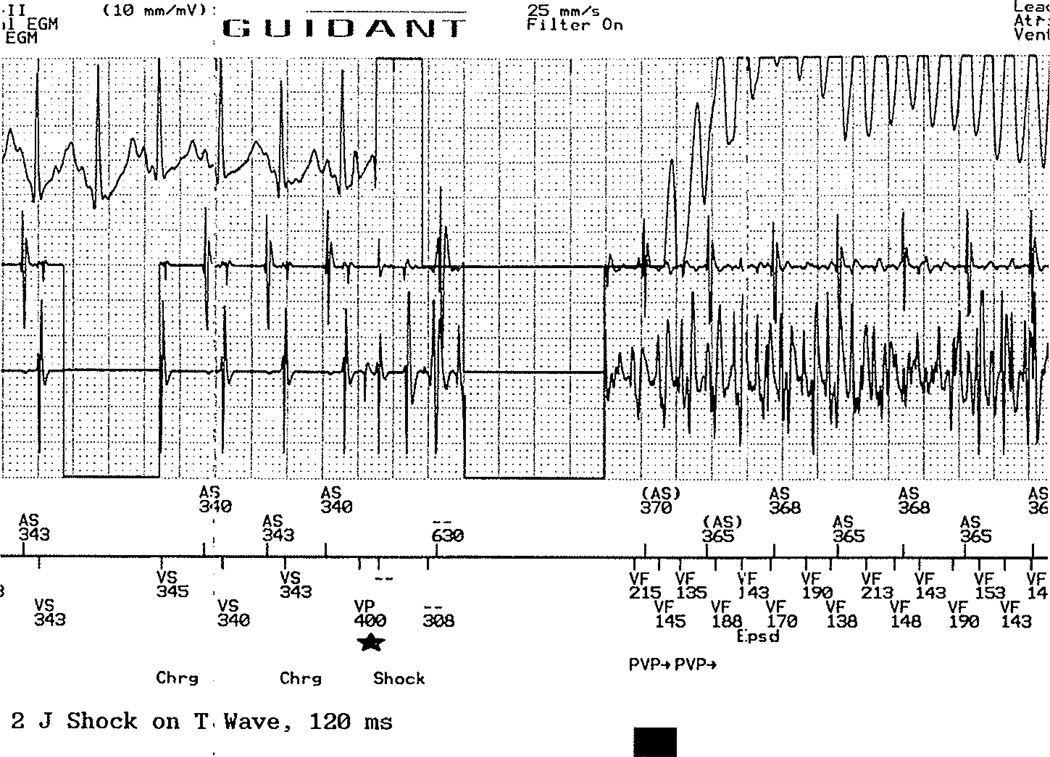
Five days following device implantation, he remained with consistent ventricular capture at rates up to 150 bpm and no episodes of TdP. All medication and pacing was held for a period of 4 h in order to reattempt ICD testing. Again, aggressive pacing protocols with shock on T following a ventricular drive train and burst pacing at baseline and following epinephrine and isoproterenol was unsuccessful. A shock on T was then administered onto the T wave of a sinus beat, which was successful in initiating TdP (Fig. 4). The device demonstrated good sensing, and the patient was successfully defibrillated with 11 J.
Fig. 4.
Introduction of shock on the T wave of a sinus beat which successfully induces Tdp. In order to program this, a single ventricular pacing spike was introduced during the refractory period of the previous sinus bear (star), with the shock delivered 120 ms later
Attempts to transition to oral feedings and antiarrhythmics resulted in abdominal distention, and colonic strictures were demonstrated by contrast studies. An exploratory laparotomy was performed which revealed strictures of the ascending colon, and a bowel resection was performed without incident. The abdomen was entered via a left lower quadrant incision in order to avoid the extraperitoneal ICD pocket.
Feeding was reattempted without difficulty, and transition to an oral regimen of medications was made. Ultimately, he was titrated to a dose of 5.5 mg/kg/dose of mexiletine TID, propranolol 4 mg/kg/dose (divided TID), and oral magnesium supplementation. He was discharged from the hospital 1 month following ICD placement.
He was followed closely as an outpatient with trans-telephonic monitoring of his ICD. He had multiple episodes of TdP and received one ICD shock. Most such episodes seemed to be preceded by sinus tachycardia. He underwent thoracoscopic lateral stellate gangliectomy which was performed at 3 months of life. Following this procedure, he had a transient increase in episodes of TdP, which resolved approximately 3 to 4 weeks after surgery. At 13 months of age, the total number of episodes of TdP appeared to be increasing despite increasing doses of mexilitene (8 mg/kg/dose). DDD pacing was converted to AAI pacing secondary to concerns that ventricular pacing was now potentially pro-arrhythmic. There was a marked diminution in the total number of episodes and shocks following this change. At 30 months of age, he has had occasional episodes of TdP with 42 total appropriate and successful ICD shocks since implant with no shocks in the past 6 months. He has minimal eyelid lag in his left eye following gangliectomy.
Genetic testing was initiated soon after birth, which confirmed the clinical suspicion of a de novo SCN5A (LQT3) 4418 T≥G nucleotide change resulting in a F1473C missense mutation. The mutation was in the DIII/DIV inter domain linker region [1]. Both parents were tested, and neither carried the SCN5A F1473C mutation.
2 Discussion
Congenital long QT syndrome (LQTS) is an inherited disorder initially reported in 1957 with the description of Jervell and Lange Nielson syndrome and subsequently Romano–Ward syndrome [2–4]. Since then, advances have been made in the understanding and management of patients with congenital LQTS. Differing risk factors for arrhythmias and sudden death have been described for patients with the various genetic forms of LQTS, allowing for tailored management and education of patients and their families [5]. KCNQ1 (LQT1), HERG (LQT2), and SCN5A (LQT3) constitute the majority of genetic defects, thus, far isolated [6].
On the basis of our patient's dramatic response to sodium channel blockade with lidocaine, a diagnosis of LQT3 was presumptively made while definitive genetic confirmation was awaited. Optimal medical management of patients with defects in SCN5A remains unclear. Although the data for LQTS in general suggest a benefit from beta blocker therapy, the usefulness for patients with LQT3 is less clear [7]. Despite this, in the absence of evidence that beta blockade is harmful in most patients with LQT3, we have treated this patient with beta blockade and ultimately proceeded with unilateral cervical sympathetic gangliectomy.
The risk of sudden death in patients with LQTS is difficult to quantify. There are data that patients who present in the newborn period with LQTS with 2:1 AV block and episodes of TdP may be at significantly elevated risk of SCD [8]. While these retrospective reviews likely suffer from selection bias, the risk of SCD in our patient was plausibly significant despite shortening of the QTc on medical therapy as this patient received multiple appropriate ICD discharges for TdP degenerating into ventricular fibrillation after discharge from the hospital. Reports of infants with defects in SCN5A and 2:1 AV block have demonstrated a significant risk of SCD, with appropriate shocks either via ICD or automated external defibrillator [9, 10]. There have been limited descriptions of ICD placement in infants less than 6 months of age. Table 1 reviews the present published data on this rare patient group. The more common approaches in the very small patient or those in whom transvenous ICD placement is contraindicated typically include epicardial lead placement with an active can and coil or patch in the subcutaneous, pericardial, or pleural spaces [11–14]. In light of the significant arrhythmia burden and high risk of sudden death despite aggressive medical management, we elected to proceed with ICD placement. This patient represents the youngest and smallest patient with a dual chamber ICD, thus far reported. He is also the youngest and smallest LQTS patient to have an ICD of any sort successfully implanted.
Table 1.
Review of all published cases of ICDs placed in infants less than 6 months
| Investigators | Patient age (months) |
Weight at surgery (kg) |
Indication for ICD | Single or dual chamber |
System characteristics |
|---|---|---|---|---|---|
| Thogersen AM, Helvind M, Jensen T et al. 2001 [11] | 2.3 | 7 | Tumor with VF | Single | Epi Sensing Lead with Subcutaneous ICD Coil |
| Greene AE, Moak JP, Di Russo G et al. 2004 [12] | 3 | 5.3 | Coronary Ischemia with VF | Single | Epi Sensing Lead with Subcutaneous Patch Electrode |
| Stephenson EA, Batra AS, Knilans TK et al.2006 [13] | 4.3 | 7 | Tumor with Arrest | Single | Epi Sensing Lead with Subcutaneous ICD Coil |
| 4.3 | 7.5 | Idiopathic VT | Single | Epi Sensing Lead with Subcutaneous ICD Coil | |
| 5 | 5.2 | LV noncompaction with Arrest | Single | Epi Sensing Lead with Subcutaneous ICD Coil | |
| Kriebel T, Ruschewski W, Gonzalez M et al. 2006 [14] | 3.2 | 4.4 | AV Canal and VT/VF | Single | Epi Sensing Leads with Pleural Space ICD Coil |
| Silver ES, Liberman L, Chung WK et al. 2009 | 1 | 3.63 | LQTS with TdP | Dual | Epi Sensing Leads with Pleural Space ICD Coil |
Pacing therapy for LQTS with 2:1 AV nodal block is a class IIA indication for placement of a pacemaker in a pediatric patient according to the 2002 ACC/AHA/NASPE recommendations [15]. Pacing has been used successfully in decreasing the incidence of tachyarrhythmias in patients with pause dependant TdP [16]. In vitro studies have demonstrated a shortened QT interval in response to rapid pacing of ventricular myocytes treated with anthopleurin, an inhibitor of the inactivation of the sodium current used to approximate LQT3 [17]. In light of this, we decided to utilize ventricular pacing with a DDD setting and a very short AV delay. The reason for the possible pro-arrhythmic effects of pacing that were shown later in his course is unclear.
As technology continues to improve and the sizes of ICDs decrease, placement of ICDs in this patient population may become more routine. In the meantime, it is the rare patient at high enough risk of SCD to be considered a candidate for neonatal ICD placement.
Acknowledgements
The authors wish to thank J. Phillip Saul, MD of the Medical College of South Carolina for his very helpful suggestions in the management of this case.
Contributor Information
Eric S. Silver, Department of Pediatrics, NY Presbyterian Hospital—Columbia University, New York, NY, USA
Leonardo Liberman, Department of Pediatrics, NY Presbyterian Hospital—Columbia University, New York, NY, USA.
Wendy K. Chung, Department of Pediatrics, NY Presbyterian Hospital—Columbia University, New York, NY, USA
Henry M. Spotnitz, Department of Surgery, NY Presbyterian Hospital—Columbia University, New York, NY, USA
Jonathan M. Chen, Department of Surgery, NY Presbyterian Hospital—Columbia University, New York, NY, USA
Michael J. Ackerman, Departments of Medicine, Pediatrics, and Molecular Pharmacology & Experimental Therapeutics/Divisions of Cardiovascular Diseases and Pediatric Cardiology, Mayo Clinic, Rochester, MN, USA
Christopher Moir, Department of Surgery, Mayo Clinic, Rochester, MN, USA.
Allan J. Hordof, Department of Pediatrics, NY Presbyterian Hospital—Columbia University, New York, NY, USA
Robert H. Pass, Email: Pediheart@aol.com, Pediatric Arrhythmia Service, Division of Pediatric Cardiology, Department of Pediatrics, Montefiore Medical Center, Albert Einstein college of Medicine, 3415 Bainbridge Avenue, Rosenthal 1, Bronx, NY 10467, USA.
References
- 1.Bankston JR, Yue M, Chung W, Spyres M, Pass RH, Silver E, et al. A novel and lethal de novo LQT-3 mutation in a newborn with distinct molecular pharmacology and therapeutic response. PLoS ONE. 2007;2:el258. doi: 10.1371/journal.pone.0001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. American Heart Journal. 1957;54:59–68. doi: 10.1016/0002-8703(57)90079-0. [DOI] [PubMed] [Google Scholar]
- 3.Ward OC. A new familial cardiac syndrome in children. Journal of the Irish Medical Association. 1964;54:103–106. [PubMed] [Google Scholar]
- 4.Romano C, Gemme G, Pongiglione R. Rare cardiac arrhythmias of the pediatric age. Ii. Syncopal attacks due to paroxysmal ventricular fibrillation. (Presentation of 1st Case in Italian Pediatric Literature) Clinica Pediatrica (Bologna) 1963;45:656–683. [PubMed] [Google Scholar]
- 5.Zareba W, Moss AJ, Schwartz PJ, Vincent GM, Robinson JL, Priori SG, et al. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. New England Journal of Medicine. 1998;339:960–965. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 6.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Priori SG. Long QT syndrome: genotype-phenotype correlations. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology. From Cell to Bedside. Philadelphia: Saunders; 2004. pp. 651–659. [Google Scholar]
- 8.Trippel DL, Parsons MK, Gillette PC. Infants with long-QT syndrome and 2: 1 atrioventricular block. American Heart Journal. 1995;130:1130–1134. doi: 10.1016/0002-8703(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 9.Divekar A, Soni R. Successful parental use of an automated external defibrillator for an infant with long-QT syndrome. Pediatrics. 2006;118:e526–e529. doi: 10.1542/peds.2006-0129. [DOI] [PubMed] [Google Scholar]
- 10.Ten Harkel AD, Witsenburg M, de Jong PL, Jordaens L, Wijman M, Wilde AA. Efficacy of an implantable cardioverter-defibrillator in a neonate with LQT3 associated arrhythmias. Europace. 2005;7:77–84. doi: 10.1016/j.eupc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Thogersen AM, Helvind M, Jensen T, Andersen JH, Jacobsen JR, Chen X. Implantable cardioverter defibrillator in a 4-month old infant with cardiac arrest associated with a vascular heart tumor. Pacing and Clinical Electrophysiology. 2001;11:1699–1700. doi: 10.1046/j.1460-9592.2001.01699.x. [DOI] [PubMed] [Google Scholar]
- 12.Greene AE, Moak JP, DiRusso G, Berger JT, Heshmat Y, Kuehl KS. Transcutaneous implantation of an internal cardioverter defibrillator in a small infant with recurrent myocardial ischemia and cardiac arrest simulating sudden infant death syndrome. Pacing and Clinical Electrophysiology. 2004;27:112–116. doi: 10.1111/j.1540-8159.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 13.Stephenson EA, Batra AS, Knilans TK, Gow RM, Gradaus R, Balaji S, et al. A multicenter experience with novel implantable cardioverter defibrillator configurations in the pediatric and congenital heart disease population. Journal of Cardiovascular Electrophysiology. 2006;17:41–46. doi: 10.1111/j.1540-8167.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 14.Kriebel T, Ruschewski W, Paul T. Implantation of an “extracardiac” internal cardioverter defibrillator in a 6-month-old infant. Zeitschrift fuÉr Kardiologie. 2005;94:415–418. doi: 10.1007/s00392-005-0236-z. [DOI] [PubMed] [Google Scholar]
- 15.Gregoratos G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) Circulation. 2002;106:2145–2161. doi: 10.1161/01.cir.0000035996.46455.09. [DOI] [PubMed] [Google Scholar]
- 16.Moss AJ, Liu JE, Gottlieb S, Locati EH, Schwartz PJ, Robinson JL. Efficacy of permanent pacing in the management of high-risk patients with long QT syndrome. Circulation. 1991;84:1524–1529. doi: 10.1161/01.cir.84.4.1524. [DOI] [PubMed] [Google Scholar]
- 17.Priori SG, Napolitano C, Cantu F, Brown AM, Schwartz PJ. Differential response to Na+ channel blockade, beta-adrenergic stimulation, and rapid pacing in a cellular model mimicking the SCN5A and HERG defects present in the long-QT syndrome. Circulation Research. 1996;78:1009–1015. doi: 10.1161/01.res.78.6.1009. [DOI] [PubMed] [Google Scholar]