Abstract
Background
An estimated 10-15% of sudden infant death syndrome (SIDS) may stem from channelopathy-mediated lethal arrhythmias. Loss of the GJA1-encoded gap junction channel protein connexin43 (Cx43) is known to underlie formation of lethal arrhythmias. GJA1 mutations have been associated with cardiac diseases including atrial fibrillation. Therefore, GJA1 is a plausible candidate gene for premature sudden death.
Methods and Results
GJA1 open reading frame mutational analysis was performed using PCR, DHPLC, and direct DNA sequencing on DNA from 292 SIDS cases. Immunofluorescence and dual whole cell patch-clamp studies were performed to determine functionality of mutant gap junctions. Immunostaining for gap junction proteins was performed on SIDS-associated paraffin-embedded cardiac tissue. Two rare, novel missense mutations, E42K and S272P, were detected in 2 of 292 SIDS cases, a 2-month-old white male and a 3-month-old white female, respectively. Analysis of the E42K victim’s parental DNA demonstrated a de novo mutation. Both mutations involved highly conserved residues and were absent in over 1000 ethnic-matched reference alleles. Immunofluorescence demonstrated no trafficking abnormalities for either mutation and S272P demonstrated wildtype junctional conductance. However, junctional conductance measurements for the E42K mutation demonstrated a loss-of-function not rescued by wildtype. Moreover, the E42K victim cardiac tissue demonstrated a mosaic immunostaining pattern for Cx43 protein.
Conclusions
This study provides the first molecular and functional evidence implicating a GJA1 mutation as a novel pathogenic substrate for SIDS. E42K-Cx43 demonstrated a trafficking-independent reduction in junctional coupling in vitro as well as demonstrating a mosaic pattern of mutational DNA distribution in deceased cardiac tissue, suggesting a novel mechanism of Cx43-associated sudden death.
Keywords: arrhythmia, connexins, death, sudden, electrophysiology, genetics
Introduction
Sudden infant death syndrome (SIDS) is defined as sudden infant death under the age of one that remains unexplained after a thorough case investigation including medical autopsy, death scene investigation and detailed review of the clinical history.1 Despite the successes of the National “Back to Sleep” Campaigns, over 2000 infants each year die from SIDS.2 Although SIDS remains poorly understood with largely unknown etiology, recent clinical and molecular evidence has implicated heritable arrhythmia syndromes as a cause of up to 10-15% of SIDS.3-8
GJA1 encodes connexin43 (Cx43), the predominant ventricular gap junction connexin and key protein in the maintenance of synchronous ventricular contraction. Mutations in GJA1 can cause oculodentodigital dysplasia (ODDD), a disease with a multi-organ presentation, which in rare cases can include cardiac abnormalities and sudden death.9 Human mutations in both connexin40 (Cx40) and Cx43 have been linked to atrial fibrillation.10, 11 Additionally, both global and cardiac-specific Cx43 knockout mice display cardiac abnormalities and sudden death.12, 13 Given that Cx43 disruption can result in cardiac arrhythmias and sudden death, and that some SIDS cases may stem from cardiac arrhythmias, we hypothesized that mutations in GJA1- encoded Cx43 may cause some cases of SIDS.
Methods
Population-based cohort of SIDS
292 SIDS cases derived from population-based cohorts as well as individually referred cases of unexplained infant deaths (114 female infants, 177 male infants, 1 unknown; 203 white, 76 African-American, 10 Hispanic, 2 Asian, 1 unknown; average age, 2.9 ± 1.9 months; range, 6 hours - 12 months) were submitted to the Mayo Clinic Windland Smith Rice Sudden Death Genomics Laboratory for post-mortem genetic testing. To be defined as SIDS, the death of the infant under age one year had to be sudden, unexpected, and unexplained following a comprehensive medico-legal autopsy.1 Infants whose death was due to asphyxia or specific disease were excluded. This study was approved by Mayo Clinic’s Institutional Review Board as an anonymous study. As such, only limited medical information was generally available, including sex, ethnicity, and age at death. Time of day, medication use, and position at death were not available. By definition, the infant’s medical history and family history were negative. In some instances, the DNA Results committee and IRB at our institution permitted the re-identification of cases with mutations that may pose a threat to the family. In such cases, participating subjects gave informed consent.
GJA1 mutational analysis
Genomic DNA was extracted from frozen necropsy tissue with the Qiagen DNeasy Tissue Kit (Qiagen, Inc, Valencia, California, USA) or from autopsy blood with the Puregene DNA Isolation Kit (Gentra, Minneapolis, Minnesota, USA). Using polymerase chain reaction (PCR), denaturing high-performance liquid chromatography (DHPLC), and direct DNA sequencing, open reading frame/splice site mutational analysis on GJA1 (chromosome 6q22.31, one coding exon) was performed as previously described.14 PCR and DHPLC conditions are available upon request. 1000 ethnic-matched adult reference alleles were examined for the absence of identified mutations. Paternity was confirmed using the ABI Verification Set (Life Technologies Corporation, Carlsbad, CA).
Plasmid construction of Cx43 expression vectors
For electrophysiologic studies, full-length coding sequence for rat Cx43 (GenBank Acc # NW_047601), which is >97% homologous to human, was subcloned into pIRES2-dsRED (Clontech, Mountain View, CA). The recombinant plasmid expressed both Cx43 and dsRED as a bicistronic mRNA, allowing connexin-expressing cells to be directly selected for electrophysiological studies. For fluorescent microscopy, rat Cx43 was subcloned into either mCHERRY or GFP (Gift from Dr. David C. Spray, Albert Einstein College of Medicine, Bronx, NY), linking the fluorescent protein to the C-terminus of Cx43. All mutations were incorporated via site-directed mutagenesis using a method adapted from the QuickChange Mutagenesis kit (Stratagene, La Jolla, CA), and vectors were sequenced to confirm the appropriate mutation. Analysis of Cx43-containing plaques in N2A cells was done by counting plaques from all images (n=3 transfections) and statistical analysis (one way ANOVA with Tukey-HSD correction) was performed using Statistica 6.0. N2A cells were transfected using Lipofectamine (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions.
Electrophysiological recordings for WT and mutant Cx43
Electrophysiological recordings of gap junction currents were obtained from N2A cell pairs transiently transfected with WT-Cx43 and/or E42K-Cx43. Junctional conductance was measured between cell pairs using dual whole cell voltage clamp with 1D patch-clamp amplifiers (Axopatch; Axon Instruments, Foster City, CA) at room temperature (RT). The bath solution contained 135 mM NaCl, 5 mM KCl, 2 mM CsCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, 5 mM dextrose, 2 mM pyruvate, and 1 mM BaCl2, pH 7.4. Patch electrodes had resistances of 3 MΩ to 5 MΩ when filled with internal solution (125 mM CsCl, 10 mM EGTA, 0.5 mM CaCl2, and 10 mM HEPES, pH 7.2). Currents were filtered at 0.5 kHz and sampled at 2 to 5 kHz. Data were acquired with appropriate software (PCLAMP8; Axon Instruments) and analyzed (PCLAMP8 [Axon Instruments] and ORIGIN 6.0 [Microcal Software, Northampton, MA]). Each cell of a pair was initially held at a common holding potential of 0 mV. To evaluate junctional coupling, 200-ms hyperpolarizing pulses from 0 mV to −20 mV were applied to one cell to establish a transjunctional voltage gradient (Vj), and junctional current was measured in the second cell (held at 0 mV). T-test was performed for relevant comparisons.
Immunostaining for intercalated disc proteins
Glass-mounted sections of formalin-fixed, paraffin embedded myocardial samples (5-7μm) were prepared by the Mayo Clinic Tissue and Cell Molecular Analysis core facility using a Leica RM2255 rotary microtome. The sections were deparaffinized, dehydrated and rehydrated by immersion as follows: 5 min in xylene, 2 min in xylene, 2 min in 100% ethanol, 2 min in 75% ethanol, 2 min in 50% ethanol, 5 min in distilled water. For antigen retrieval, sections were placed in citrate buffer (pH 6.0), brought to a boil (11 min microwaving), left to cool to RT, and washed in PBS (5 min). To reduce background auto-fluorescence, samples were simultaneously blocked with 3% goat serum, 1% BSA and 0.5% Triton X 100 in PBS for 40 min at RT. Primary antibody incubation (diluted in blocking buffer) occurred at 4°C overnight. After equilibrating at RT, sections were washed 3x in PBS for 5 min, and incubated with Cy3-conjugated goat anti-mouse or anti-rabbit IgG (H+L) secondary antibodies (1:400 in PBS, Jackson Immunolabs, West Grove, PA) for 2 hrs at RT. Samples were washed 3x in PBS for 5 min, and mounted in 50% glycerol, 50% PBS and 0.1% propyl gallate.
Chemical reagents
Antibodies were as follows: rabbit polyclonal anti-connexin43 (Sigma-Aldrich, St. Louis, MO, 1:400), mouse monoclonal anti-Pan cadherin (Sigma, 1:400), mouse monoclonal anti-plakoglobin (Sigma, 1:1000), mouse monoclonal anti-plakophilin2 (2a+2b, Fitzgerald Industries International, Acton, MA, undiluted), mouse monoclonal anti-desmoplakin (Fitzgerald, 1:10), rabbit polyclonal anti-ZO-1 (Santa Cruz Biotechnology, Santa Cruz, CA, 1:100).
Results
Identified Mutations
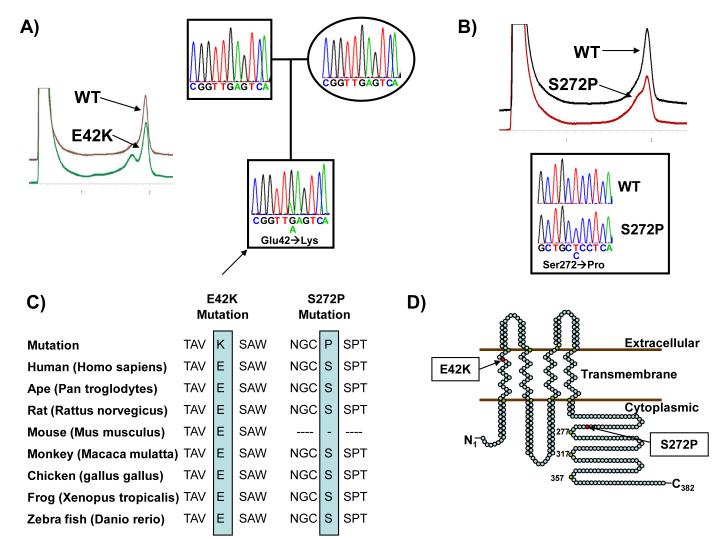
Overall, mutational analysis of GJA1 revealed two novel missense mutations in 2 out of 292 SIDS victims (<1%). E42K-Cx43 was identified in a two-month-old white male (Figure 1A) and S272P-Cx43 in a three-month-old white female (Figure 1B). Both mutations were absent in over 1000 ethnic-matched control alleles and involved residues highly conserved across a variety of species (Figure 1C). E42K-Cx43 localized to the extracellular side of the first transmembrane domain, while S272P-Cx43 localized to the C-terminus (Figure 1D). The common polymorphism A253V-Cx43 was similarly found in both cases and controls.
Figure 1.
Identification of GJA1 mutations in a SIDS cohort. (A) DHPLC profiles (normal, brown and abnormal, green trace) and DNA sequencing chromatograms for affected GJA1-E42K proband (arrow) along with his genotype-negative parents. (B) DHPLC profiles (normal, black and abnormal, red trace) and DNA sequencing chromatogram for GJA1-S272P compared with normal. (C) Sequence conservation is compared for both mutations. (D) Illustrated is the linear topology of Cx43 with mutation localization. Numbering on the protein refers to the amino acid at that location.
E42K Case Description
In the case of the E42K-positive infant, we were able to unblind the case and obtain more information. The E42K-positive SIDS victim was born 4lbs, 11oz at 34 weeks along with a fraternal twin brother and spent a few days in the NICU after birth. No ECG was available from this stay. Over the next two months, his mother noticed times that he would be very sleepy or fall asleep immediately after feedings while his twin brother would remain wide awake. His parents also noticed times while sleeping that his skin became slightly pale and almost translucent. During the last two weeks of his life, his parents had to constantly wake him or use a cold compress to keep him awake long enough to eat. Interestingly, the family history is positive for previous infant deaths in twins but these deaths are three generations removed from the current victim. Neither parent has cardiac abnormalities and both were negative for the mutation (Figure 1A). Paternity was confirmed. The other three siblings of the victim remain in good health. Poor tissue quality precluded our ability to extract DNA from other tissue types to confirm mosaicism.
Trafficking of Connexin43 Mutations
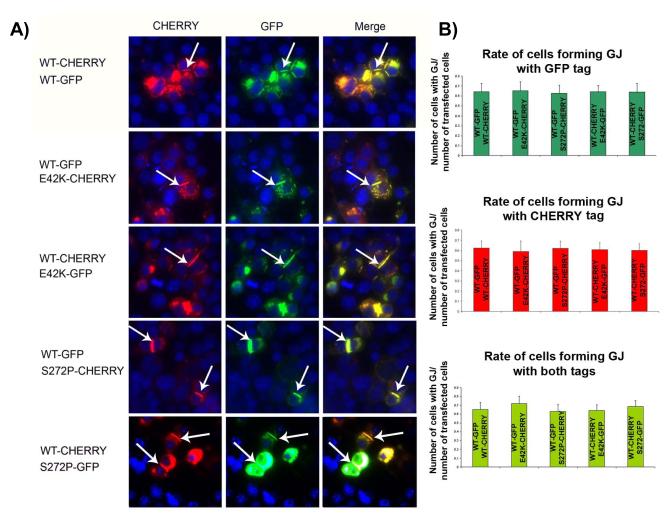
Given that loss of trafficking is a common pathogenic mechanism in connexin mutations,15-17 we investigated the effects of E42K- and S272P-Cx43 on Cx43 trafficking. To address this, we transfected Cx43-negative neuroblastoma (N2A) cells with wildtype (WT)-, E42K- or S272P-Cx43 tagged either with GFP or mCHERRY and assessed the number of plaques formed between the cells. No defects in trafficking with mutant Cx43 alone were identified (data not shown). To determine the effects of the mutants on wildtype Cx43 trafficking in an effort to recapitulate the heterozyogous state of the infant, we co-transfected N2A cells with WT and mutant Cx43 together. The number of gap junctional plaques did not differ in cells containing either of the Cx43 mutations as compared to WT and co-transfection of WT Cx43 together with E42K-Cx43 or S272P-Cx43 did not alter the rate of plaque formation, indicating that there are other mechanisms independent from trafficking by which these mutations affects cardiac conduction (Figure 2).
Figure 2.
E42K-Cx43 and S272P-Cx43 are trafficked to the cellular membrane as well as WT-Cx43. (A) Representative co-transfected cells with the formation of gap junctional (GJ) plaques (arrow heads) in both cases of E42K and S272P mutations, which fully overlay with WT-Cx43 indicating unaltered trafficking of mutated Cx43 as well as WT-Cx43 in presence of mutated Cx43 in the cell (DAPI staining in blue). (B) Quantification of plaque formation relative to the number of cells transfected. No difference in plaque formation per transfected cell can be observed (p>0.05 for all comparisons).
Electrophysiologic Properties of Connexin43 Mutations
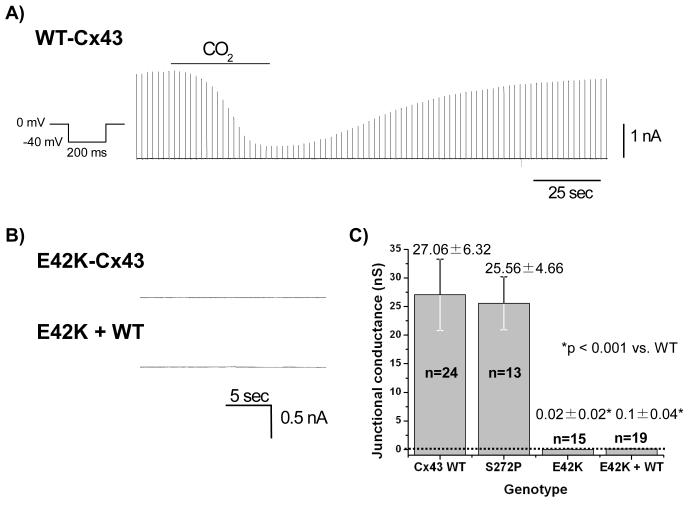
To further evaluate the functional integrity of the mutant gap junctions, we performed dual whole cell patch clamp. Cx43 constructs were expressed in N2A cells and junctional currents were recorded from cell pairs 48 hours after transfection. Cell pairs expressing WT-Cx43 robustly coupled (27.06 ± 6.32 nS, n=24, Figure 3A&C). However, cells expressing the E42K-Cx43 mutation displayed a complete absence of electrical coupling (Gj values were 0.02 ± 0.02 nS, n=15, p<0.001 vs. WT-Cx43, Figure 3B&C). To assess whether or not mutant Cx43 decreased wildtype junctional currents, cells were co-transfected with WT- and E42K-Cx43. Gj values were 0.1 ± 0.04, n=19, p<0.001 vs. WT-Cx43, indicating a complete failure of WT-Cx43 to rescue the phenotype brought about by E42K (Figure 3B&C). Additional electrophysiologic experiments confirmed the GFP and mCHERRY tags did not alter the E42K effects on the connexin protein (data not shown). In contrast to the E42K phenotype, S272P-expressing cells demonstrated wildtype coupling (p>0.05 vs. WT-Cx43, Figure 3C).
Figure 3.
E42K-Cx43 but not S272P-Cx43 demonstrates loss of intercellular coupling. (A) Representative dual whole cell voltage clamp traces for N2A cells expressing WT-Cx43 alone, E42K-Cx43 alone or E42K- and WT-Cx43 together. WT-Cx43-expressing cells demonstrated robust connexin-mediated intercellular coupling when submitted to the protocol shown. This current was responsive to CO2 indicating coupling is connexin-mediated. (B) In contrast, both E42K-Cx43 alone and together with WT-Cx43 demonstrated no intercellular coupling. (C) Shown from left to right are the junctional conductances for WT-, S272P-, E42K-alone and E42K-Cx43 with WT-Cx43 in N2A cells. Dotted line denotes 0 nS conductance.
Immunohistochemical Staining of SIDS-Associated Cardiac Tissue
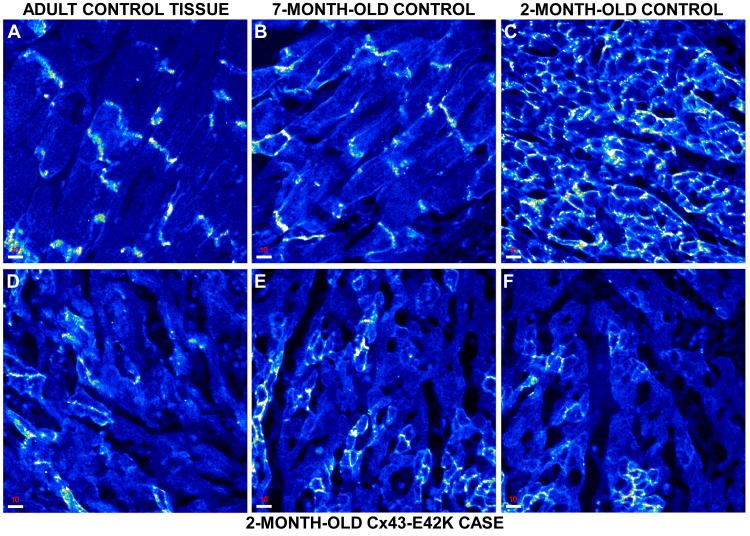
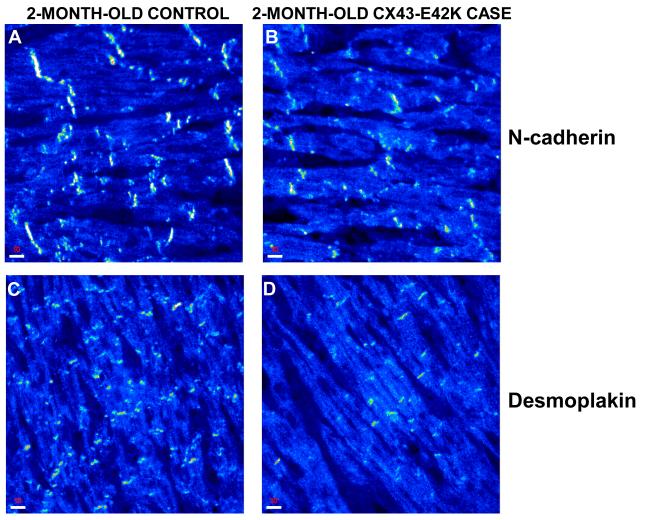
Given its abnormal function in vitro, we performed immunohistochemistry on myocardial tissue from the proband to investigate the effects of E42K on the localization of Cx43 as well as on the distribution patterns of further intercalated disc components (including N-cadherin, desmoplakin, plakoglobin, plakophilin-2 and ZO-1). Ventricular samples obtained at autopsy from an adult, a 7-month-old, and a 2-month-old with no clinical or pathological evidence of heart disease were subjected to the same immunostaining protocol and served as controls. Intercalated discs showed strong immunoreactive signal for Cx43 in both the adult and 7-month-old myocardial samples (Figure 4A&B). In contrast, Cx43 showed a different, rather polygonal distribution pattern in the 2-month-old myocardial specimen, staining uniformly the entire cardiac myocyte membrane instead of clustering at intercalated disc sites (Figure 4C). Our observations are in agreement with previous observations.18 In marked contrast to the control samples, the E42K-Cx43 SIDS case demonstrated a patchy distribution pattern for Cx43. Certain areas seemed to express Cx43 at control levels, while adjacent areas showed no immunoreactive signal for the gap junction protein (Figure 4D-F). These observations suggest somatic mosaicism. Signal for both the non-desmosomal adhesion molecule N-cadherin and the plakin protein demoplakin was strong and indistinguishable from controls, demonstrating an adult-like signal clustering at intercalated discs (Figure 5). Immunoreactive signal for plakoglobin, plakophillin-2 and ZO-1 was absent from both the SIDS and the 2-month-old control sample (data not shown). It is noteworthy that lack of immunoreactive signal does not necessarily indicate the absence of protein in the myocardium at this stage. It suggests, however, that the expression levels and/or localization patterns are such that the protein cannot be detected by this approach.
Figure 4.
Confocal images of Cx43 immunostaining in case and control cardiac tissue. (A, B) Adult and 7-month-old control myocardium with concentrated Cx43 staining at the intercalated discs. Scale bars, 10 μm. (C) 2-month-old control staining with spatially diffuse pattern. Scale bar, 10 μm. (D-F) 2-month-old E42K-Cx43 infant with concentrated areas of wildtype staining and areas of complete loss of signal. Scale bars, 10 μm.
Figure 5.
Normal N-cadherin and desmoplakin staining in control and case. Confocal images of control and E42K-Cx43 myocardium immunostained for N-cadherin (top) and desmoplakin (bottom). (A, C) 2-month-old control myocardium staining, while (B, D) 2-month-old E42K-Cx43 case myocardium staining. Scale bars, 10 μm.
Discussion
This is the first study to report a SIDS-associated mutation in GJA1-encoded Cx43 that causes formation of a biophysically non-functional gap junction channel. We discovered two novel Cx43 mutations in two unrelated SIDS victims, with both mutations involving highly conserved residues and absent in over 1000 ethnic-matched controls. The first, E42K, localizes to the transmembrane region near the extracellular loops, which are important for proper formation of fully functioning connexons.19 S272P localizes to the cytoplasmic C-terminus, near a region of multiple serine phosphorylation sites.19 However, functional studies for S272P showed wildtype trafficking and gap junctional coupling, whereas E42K demonstrated a profound reduction in junctional conductance for cells expressing either the E42K-Cx43 mutation alone or together with WT, similar to the phenotype of other disease-associated connexin mutations.11, 20 In addition, immunostaining of the decedent’s tissue revealed patchy loss of Cx43, suggestive of somatic mosaicism similar to that seen in cases of atrial fibrillation induced by somatic connexin 40 and 43 mutations10, 11 and highly predictive of an arrhythmogenic substrate. We predict therefore that while the death of the E42K-positive case may be directly due to mutation-induced pathology, the death of the S272P-positive case remains unexplained, since our investigations demonstrated wildtype junctional physiology with this second case.
Functional gap junction channels are formed by two hemichannels (connexons) in adjacent cells, each consisting of a hexamer of one or more types of connexin protein subunits, and allow for intercellular communication via the passage of electrical impulses between myocytes and thus facilitate synchronous contraction of the myocardium.21, 22 Cx43 is the major gap junction protein in ventricular tissue.21 Global deletion of the GJA1 gene in mice results in death shortly after birth, primarily due to structural abnormalities in the cardiac outflow tract present well before birth.12, 13 In contrast, Cx43 conditional knockout mice (CKO) show no significant differences in their phenotype or behavior compared to their control littermates and yet die suddenly at 81.4 ± 3.3 days.23 As shown by immunohistochemistry and Western immunoblotting studies, by 45 days, Cx43 expression in the ventricles was reduced to 18% in the CKO animals compared to controls. High power views showed areas where cells formed rare plaques alternating with clusters of Cx43-positively stained cells. The QRS amplitude was progressively decreased in the CKO animals and paralleled the loss of Cx43 expression. Programmed electrical stimulation induced sustained polymorphic VT in 8 of 10 CKO animals at 45 days, while only non-sustained VT was induced in only 1/3 of the control mice. Yet, despite the increased susceptibility of CKO mice to lethal arrhythmias, hemodynamic parameters and ventricular contractility of CKO mice at all time points was no different to controls.23
Moreover, chimeric Cx43 mice, which were generated by introducing Cx43-deficient embryonic stem cells in wildtype recipient blastocysts, also demonstrated conduction delay and spontaneous ventricular tachycardia in the absence of structural changes.24 Gap junction expression in those hearts was highly abnormal with foci of Cx43-deficient myocytes interspersed throughout an otherwise well-coupled myocardial syncitium.25 Telemetry recordings in the chimeric animals showed increased frequency of premature ventricular contractions compared to control littermates. Moreover, multiple morphologies of ventricular ectopy were recorded suggesting the presence of multiple arrhythmogenic foci. It is true that the chimeric animals showed decreased ventricular systolic function (fractional shortening was 33.2 ± 2.08% in the chimeric animals compared to 41.0 ± 1.27% in controls). However, although the LV systolic pressures were decreased in the chimeras, there were no differences in the diastolic pressures between chimeric and control animals. Moreover, there was no evidence of ventricular dilatation or hypertrophy.25 Taken together, these results suggest that gap junction remodeling predisposes to arrhythmias but does not obligate any significant mechanical dysfunction. It is likely that the cardiac tissue in our decent mimics this latter instance, and such findings in murine models suggest why no cardiac abnormalities were detected at autopsy to render a non-SIDS diagnosis and why the infant was able to survive two months before death.
Loss of ventricular gap junction function underlies formation of an arrhythmogenic substrate in animal models.24, 26, 27 However, as the previously discussed studies support, it is likely that heterogenous Cx43 loss, not reduction of Cx43 per se, creates an arrhythmogenic substrate whereby safe, albeit slow, conduction is dissipated and disrupted by sinks of well-coupled cells. Thus the spontaneous ventricular tachyarrhythmias and sudden death noted in cardiac-restricted knockout mice may actually stem from low residual mosaic expression of Cx43. The pro-arrhythmic nature of heterogeneous Cx43 expression is further supported by the observation that heterogeneous populations of neonatal murine ventricular myocytes have pro-arrhythmic impulse propagation.28
Moreover, Boulaksil et al. demonstrated increased heterogeneity of Cx43 in both congestive heart failure patients with documented ventricular tachycardias as well as in a heart failure mouse model which showed inducible polymorphic VT in the setting of heterogeneous Cx43 loss.29 In this case, whether the heterogeneity of Cx43 is related to the polymorphic morphology of the tachycardias remains unsolved. Lastly, a Cx43 somatic mutation was recently found to underlie lone atrial fibrillation in a patient.11 Immunohistochemistry of the patient’s atrial tissue showed a mosaic pattern of intracellular retention of Cx43 and a mosaic pattern of aberrant gap junction formation, very much like the images we obtained from our case. Expression of the mutant Cx43 form in oocyte pairs from the genus Xenopus showed a dramatic decrease in cell-cell coupling conductance.11
We hypothesize, given the immunostaining pattern of the decedent, that mutant E42K-Cx43, although trafficking properly in an in vitro setting, does not properly interact with adjacent Cx43 to form fully functional gap junctions, forming an arrhythmogenic substrate. We know that other cardiac disorders trigger reduction in Cx43 expression,30, 31 and it is likely that such changes may not be detected at levels assessed by our plaque formation assay. Further, our immunostaining assay is optimized to detect high levels of Cx43 at gap junctions.32 A change in expression may reduce Cx43 to a level undetectable by our current assay. This may explain why our in vitro data in N2A cells demonstrates proper trafficking whereas intact decedent heart tissue demonstrates a mosaic pattern.
Since this infant was not wearing a Holter monitor at the time of his tragic demise, it is impossible to verify our postulated exit rhythm of ventricular fibrillation. Nevertheless, E42K-Cx43 was identified in a sudden death cohort, in a gene with known association with cardiac disease, was located in a highly conserved region of the gene, and absent in over 1000 ethnic-matched alleles. In addition, the heterogeneous staining pattern of the infant’s cardiac tissue offers strong evidence that this mutation conferred a sudden death predisposing arrhythmogenic phenotype. Further, this deceased infant’s loss-of-function E42K-Cx43 mutation and likely somatic mosaicism essentially represents the human equivalent of the chimeric Cx43 mouse. The S272P-Cx43 mutation had a wildtype phenotype in our investigations and therefore, although rare and highly conserved, it cannot at this time be considered a sudden-death predisposing mutation.
In conclusion, this is the first study to implicate a mutation in a connexin protein in the pathogenesis of SIDS. E42K-Cx43 demonstrated a severe loss-of-function phenotype, and is located in a crucial conserved region of the protein. While such SIDS-associated Cx43 mutations are rare, this study provides evidence for the contribution of Cx43 dysregulation to sudden death, and contributes to the growing body of literature implicating cardiac arrhythmias as the cause for a subset of SIDS.
Acknowledgments
We thank Dr. David C. Spray of Albert Einstein College of Medicine for plasmids used in the transfection studies.
Funding Sources: This work was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program (M.J.A.), The FDA Spring 2010 Postdoctoral Fellowship from the American Heart Association (to A.A.) and grants EY13869 (to M.S.), HL102361 (to J.E.S.), HL083205 (to H.S.D.), and HD42569 (to M.J.A.) from National Institutes of Health, USA.
Footnotes
Conflict of Interest Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krous HF, Beckwith JB, Byard RW, Rognum TO, Bajanowski T, Corey T, Cutz E, Hanzlick R, Keens TG, Mitchell EA. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114:234–238. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 2.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2004 period linked birth/infant death data set. Natl Vital Stat Rep. 2007;55:1–32. [PubMed] [Google Scholar]
- 3.Tester DJ, Ackerman MJ. Sudden infant death syndrome: how significant are the cardiac channelopathies? Cardiovasc Res. 2005;67:388–396. doi: 10.1016/j.cardiores.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Tester DJ, Dura M, Carturan E, Reiken S, Wronska A, Marks AR, Ackerman MJ. A mechanism for sudden infant death syndrome (SIDS): stress-induced leak via ryanodine receptors. Heart Rhythm. 2007;4:733–739. doi: 10.1016/j.hrthm.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz PJ, Stramba-Badiale M, Segantini A, Austoni P, Bosi G, Giorgetti R, Grancini F, Marni ED, Perticone F, Rosti D, Salice P. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338:1709–1714. doi: 10.1056/NEJM199806113382401. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz PJ, Priori SG, Dumaine R, Napolitano C, Antzelevitch C, Stramba-Badiale M, Richard TA, Berti MR, Bloise R. A molecular link between the sudden infant death syndrome and the long-QT syndrome. N Engl J Med. 2000;343:262–267. doi: 10.1056/NEJM200007273430405. [DOI] [PubMed] [Google Scholar]
- 7.Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, Menendez TM, Brugada J, Pollevick GD, Wolpert C, Burashnikov E, Matsuo K, Wu YS, Guerchicoff A, Bianchi F, Giustetto C, Schimpf R, Brugada P, Antzelevitch C. Sudden death associated with short-QT syndrome linked mutations in HERG. Circulation. 2004;109:30–35. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 8.Arnestad M, Crotti L, Rognum TO, Insolia R, Pedrazzini M, Ferrandi C, Vege A, Wang DW, Rhodes TE, George AL, Jr, Schwartz PJ. Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation. 2007;115:361–367. doi: 10.1161/CIRCULATIONAHA.106.658021. [DOI] [PubMed] [Google Scholar]
- 9.Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gollob MH, Jones DL, Krahn AD, Danis L, Gong XQ, Shao Q, Liu X, Veinot JP, Tang AS, Stewart AFR, Tesson F, Klein GJ, Yee R, Skanes AC, Guiraudon GM, Ebihara L, Bai D. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354:2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- 11.Thibodeau IL, Xu J, Li Q, Liu G, Lam K, Veinot JP, Birnie DH, Jones DL, Krahn AD, Lemery R, Nicholson BJ, Gollob MH. Paradigm of genetic mosaicism and lone atrial fibrillation: physiological characterization of a connexin 43-deletion mutant identified from atrial tissue. Circulation. 2010;122:236–244. doi: 10.1161/CIRCULATIONAHA.110.961227. [DOI] [PubMed] [Google Scholar]
- 12.Dobrowolski R, Willecke K. Connexin-caused genetic diseases and corresponding mouse models. Antioxid Redox Sign. 2009;11:283–296. doi: 10.1089/ars.2008.2128. [DOI] [PubMed] [Google Scholar]
- 13.Eckardt D, Kirchhoff S, Kim JS, Degen J, Theis M, Ott T, Wiesmann F, Doevendans PA, Lamers WH, de Bakker JM, van Rijen HV, Schneider MD, Willecke K. Cardiomyocyte-restricted deletion of connexin43 during mouse development. J Mol Cell Cardiol. 2006;41:963–971. doi: 10.1016/j.yjmcc.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Ackerman MJ, Tester DJ, Jones G, Will MK, Burrow CR, Curran M. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc. 2003;78:1479–1487. doi: 10.4065/78.12.1479. [DOI] [PubMed] [Google Scholar]
- 15.Deschenes SM, Walcott JL, Wexler TL, Scherer SS, Fischbeck KH. Altered trafficking of mutant connexin32. J Neurosci. 1997;17:9077–9084. doi: 10.1523/JNEUROSCI.17-23-09077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roscoe W, Veitch GIL, Gong XQ, Pellegrino E, Bai D, McLachlan E, Shao Q, Kidder GM, Laird DW. Oculodentodigital dysplasia-causing connexin43 mutants are non-functional and exhibit dominant effects on wild-type connexin43. J Biol Chem. 2005;280:11458–11466. doi: 10.1074/jbc.M409564200. [DOI] [PubMed] [Google Scholar]
- 17.Tong D, Colley D, Thoo R, Li TY, Plante I, Laird DW, Bai D, Kidder GM. Oogenesis defects in a mutant mouse model of oculodentodigital dysplasia. Dis Model Mech. 2009;2:157–167. doi: 10.1242/dmm.000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation. 1994;90:713–725. doi: 10.1161/01.cir.90.2.713. [DOI] [PubMed] [Google Scholar]
- 19.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell B. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha J, N, Jabs EW. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- 21.Duffy HS, Fort AG, Spray DC. Cardiac connexins: genes to nexus. Adv Cardiol. 2006;42:1–17. doi: 10.1159/000092550. [DOI] [PubMed] [Google Scholar]
- 22.Hervé JC, Bourmeyster N, Sarrouilhe D, Duffy HS. Gap junctional complexes: from partners to functions. Prog Biophys Mol Biol. 2007;94:29–65. doi: 10.1016/j.pbiomolbio.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res. 2004;95:1035–1041. doi: 10.1161/01.RES.0000148664.33695.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutstein DE, Morley GE, Vaidya D, Liu F, Chen FL, Stuhlmann H, Fishman GI. Heterogeneous expression of gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194–1199. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- 25.Gutstein DE, Danik SB, Lewitton S, France D, Liu F, Chen FL, Zhang J, Ghodsi N, Morley GE, Fishman GI. Focal gap junction uncoupling and spontaneous ventricular ectopy. Am J Physiol Heart Circ Physiol. 2005;289:H1091–H1098. doi: 10.1152/ajpheart.00095.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters NS. Gap junctions and clinical cardiology: from molecular biology to molecular medicine. Eur Heart J. 1997;18:1697–1702. doi: 10.1093/oxfordjournals.eurheartj.a015162. [DOI] [PubMed] [Google Scholar]
- 27.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleber AG, Beauchamp P, Li W, Rigoli G, Saffitz JE. Effect of heterogeneous connexin expression on impulse propagation in cultured strands of murine ventricular myocytes. Heart Rhythm. 2010;7:S160. [Google Scholar]
- 29.Boulaksil M, Winckels SKG, Engelen MA, Stein M, van Veen TA, Jansen JA, Linnenbank AC, Bierhuizen MF, Groenewegen WA, van Oosterhout MF, Kirkels JH, de Jonge N, Varró A, Vos MA, de Bakker JM, van Rijen HV. Heterogeneous connexin43 distribution in heart failure is associated with dispersed conduction and enhanced susceptibility to ventricular arrhythmias. Eur J Heart Fail. 2010;12:913–921. doi: 10.1093/eurjhf/hfq092. [DOI] [PubMed] [Google Scholar]
- 30.Nattel S, Maguy A, Bouter S Le, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 31.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88:864–875. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 32.Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]