SUMMARY
Background
A young adult female presented with syncope and periodic weakness. A 12-lead electrocardiogram showed frequent premature ventricular contractions and prolonged QU interval. Repetitive runs of nonsustained ventricular tachycardia were recorded at night.
Investigations
Electromyography, muscle biopsy, MRI, echocardiography, exercise stress testing using Bruce protocol with microvolt T-wave alternans testing, 24 h Holter monitoring, electrophysiological testing and examination of the effects of sleep and sleep stage on the patient's ventricular arrhythmias.
Diagnosis
Type 1 Andersen–Tawil syndrome, (also known as type 7 long QT syndrome). Severe ventricular arrhythmia was observed, predominantly during rapid eye movement sleep. We speculate that the autonomic instability present during rapid eye movement sleep precipitates increasing vulnerability to sleep-related ventricular tachycardia.
Management
β-blocker therapy alone, subsequently combined with mexiletine treatment.
Keywords: Andersen–Tawil syndrome, autonomic function, REM sleep, sudden cardiac death, ventricular arrhythmias
THE CASE
Thirteen years ago, a 16-year-old female presented at hospital following her first episode of syncope, which had occurred while walking. She had experienced six isolated episodes of periodic weakness over the preceding year. These primarily involved the lower extremities, which would give way, causing her to fall over. Her medical history was otherwise unremarkable, as was that of her family. A neurological work-up, which included brain and spine MRI, electro myography and muscle biopsy, was negative. With the exception of an irregular pulse, the patient's physical examination was normal.
Several years later, a clinical diagnosis of Andersen–Tawil syndrome (ATS) was considered, on the basis of the patient's history of periodic weakness, the observation of a small mandible and wide nasal root upon physical examination, and a 12-lead electrocardiogram characteristic of the condition (Figure 1). Genetic analysis using denaturing high performance liquid chromatography and direct DNA sequencing identified a mutation in the potassium inward rectifier channel, subfamily J, member 2 gene (KCNJ2), thereby establishing both the clinical and genetic diagnosis of type 1 ATS (ATS1). This mutation led to an amino acid substitution at position 75, with threo-nine replaced by methionine (Thr75Met). Laboratory functional analyses, involving heterologous expression and whole cell electro-physiological recordings of the mutant channel in mammalian cells, characterized this amino acid change as a dominant-negative, loss-of-function mutation (data not shown). The patient tested negative for all known long QT syndrome (LQTS) susceptibility gene mutations besides KCNJ2. Since ATS1 is a genetic disease that is often associated with autosomal dominant transmission, confirmatory genetic testing of the patient's parents and siblings was performed. None had the mutation, indicating that the patient's germline mutation was spontaneous (i.e. sporadic de novo).
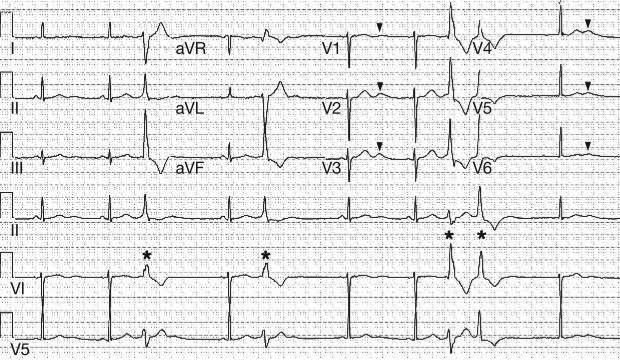
Figure 1.
A standard 12-lead electrocardiogram, characteristic of Andersen–Tawil syndrome. A prolonged QU interval with a prominent U-wave (indicated by arrowheads) is evident, along with the presence of ventricular ectopy (indicated by asterisks).
Echocardiography and a conventional electro-physiological study were unremarkable. An exercise stress test, using the Bruce protocol with microvolt T-wave alternans testing, was normal. Importantly, no exercise-induced ectopy was seen, even during maximum exertion. Ambulatory electrocardiographic recording was also performed using a 24 h Holter monitor, which recorded a total of 68,000 ectopic beats. These occurred throughout the 24 h period, but predominantly in the evening and at night. Nine runs of nonsustained ventricular tachycardia occurred at night. β-blocker therapy (atenolol—50 mg in the morning, 25 mg in the evening) was initiated in an attempt to suppress the patient's ventricular ectopy. Atenolol decreased the number of ectopic beats, but she continued to have runs of nonsustained ventricular tachycardia, particularly at night.
Since Holter monitoring had recorded severe nocturnal ventricular ectopy, and because of the association between cardiac channelopathies and the risk of sudden cardiac death (SCD) during sleep, a sleep study (polysomnography), approved by the Institutional Review Board, was performed to examine the effects of sleep and sleep stage on the patient's ventricular arrhythmo genesis while receiving β-blocker therapy. The sleep study demonstrated that the patient had no breathing abnormalities. Similar to data acquired from prior ambulatory 24 h electrocardiographic monitoring, frequent ventricular ectopy (singles, couplets and triplets, along with nonsustained ventricular tachycardia) occurred while the patient slept. Strikingly, premature ventricular contractions (PVCs) occurred only sporadically during non-rapid-eye-movement (REM) sleep. By contrast, PVCs occurred frequently during REM sleep, including couplets, triplets, and four beats of nonsustained monomorphic ventricular tachycardia (Figure 2).
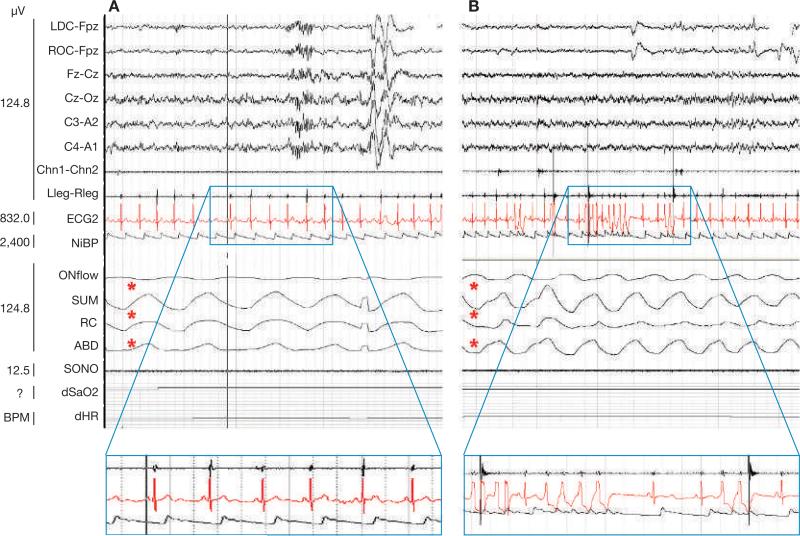
Figure 2.
Polysomnograms from the patient. (A) During non-REM sleep. A normal breathing pattern (thorax and abdominal movements and airflow present—denoted by red asterisks) and sinus rhythm (left blue rectangle) can be seen. (B) During REM sleep. A normal breathing pattern can be seen (red asterisks), but there is frequent ventricular ectopy (single premature ventricular contractions, couplets and a brief run of nonsustained monomorphic ventricular tachycardia—right blue rectangle). Abbreviation: REM, rapid eye movement.
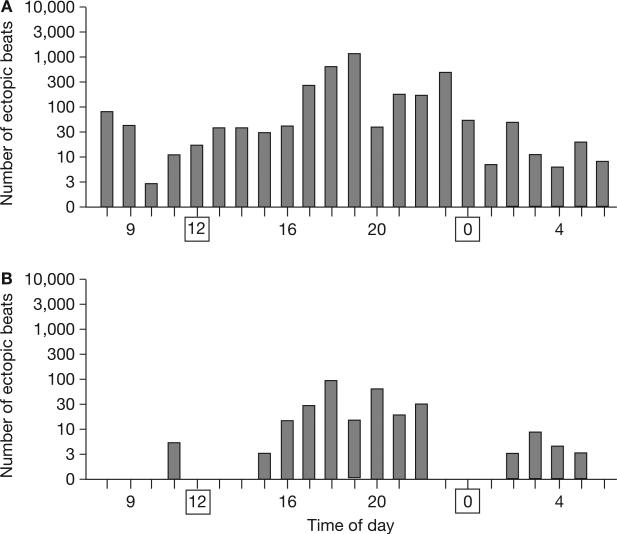
Mexiletine (an antiarrhythmic agent also used in periodic paralysis disorders) was subsequently added to the patient's treatment regime at a dosage of 250 mg three times a day. This combined therapy of β-blockers with mexiletine suppressed the ventricular ectopy dramatically, from over 4,000 ectopic beats (64 runs of ventricular tachycardia—the longest run being 10 beats) with β-blocker therapy alone (Figure 3A), to less than 300 (without ventricular tachycardias; Figure 3B), as measured by Holter monitoring. As mexiletine is not standard therapy for patients with ATS1, the principle of Koch's postulates was followed—the patient underwent trial periods with and without mexiletine, demonstrating recurrence of the profound ventricular ectopy when the drug was stopped. The patient has since continued on this combined pharmaco-therapy of β-blockers and mexiletine for four years, and remains asymptomatic as assessed by semiannual follow-up evaluations.
Figure 3.
Twenty-four hour Holter monitoring demonstrating the patient's ventricular ectopy. Midnight and noon have been marked with squares. (A) During β-blocker therapy alone. (B) During combined mexiletine and β-blocker therapy.
DISCUSSION OF DIAGNOSIS
ATS is a potassium-sensitive disorder that causes periodic muscle paralysis. The complete triad of ATS features comprises muscle weakness due to periodic paralysis, dysmorphic features (low-set ears, micrognathia, a wide nose and clinodactyly) and QU interval prolongation with ventricular arrhythmias.1 The only gene to be implicated in the condition so far—KCNJ2—encodes the potassium channel protein Kir2.1, and is linked to chromosome 17q23. This genotype has been labeled ATS1 and to date encompasses approximately 50% of patients with a clinical diagnosis of ATS.2–4 The other half of patients with ATS remain genetically elusive, and thus far there are no clinically distinguishing features between KCNJ2 genotype-positive (ATS1) and genotype-negative ATS.
More than 20 different KCNJ2 mutations have been implicated in ATS1, with variable penetrance and expressivity. Although ATS1 is the preferred nomenclature, loss-of-function mutations in KCNJ2 have also been referred to as type 7 long QT syndrome (LQT7). The consequence of KCNJ2 loss-of-function mutations is a homogeneous prolongation of transmembrane action potentials, with no increase in transmural dispersion of repolarization. The latter effect could account for the relatively benign nature of this syndrome compared with other, more typical forms, of LQTS.5
Patients with congenital LQTS can experience asymptomatic longevity, or present with a sentinel episode of syncope, seizures, and/or SCD associated with a lethal ventricular tachyarrhythmia.6 For LQT1 and LQT2, β-blocker therapy is the standard treatment, and is extremely effective. In the present case, however, substantial ventricular ectopy persisted during the day and night, despite β-blocker therapy. Certain types of congenital LQTS, such as LQT2 and LQT3, have been associated with nocturnal SCD. It was interesting, therefore, to elucidate whether the increased ventricular ectopy could be related to an occult sleep disorder or sleep stage. No sleep-related breathing disorders were detected on polysomnography, but surprisingly, the study showed that ventricular ectopy occurred predominantly during REM sleep.
During REM sleep, there is profound vascular sympathetic activation and fluctuations in cardiac autonomic drive.7 Sympathetic activation precipitates ventricular arrhythmias and might explain the ventricular arrhythmogenesis seen during sleep. In this particular case, however, ventricular ectopy cannot be explained entirely by sympathetic activation during REM sleep, since PVCs were absent in numerous treadmill tests even with maximum exercise—a powerful sympathetic stimulus. It is probably not, therefore, simply an adrenergic drive to the heart, but rather the autonomic instability that is present during REM sleep8 (intermittent bursts of sympathetic activity associated with brief but powerful bursts of para sympathetic activity) that might have contributed to the ventricular ectopy observed in this patient. In fact, ventricular arrhythmogenesis has been described in animals during periods of intense vagus nerve excitation, alongside heightened adrenergic activity.9
This additional parasympathetic mechanism might also explain why β-blockers, which suppress sympathetic stimulation, did not totally alleviate ventricular ectopy in this patient when administered alone. In some congenital forms of LQTS, such as LQT1, ventricular tachycardia commonly develops during adrenergic stimulation, such as that experienced during exertion. By contrast, more events occur during sleep or arousal in patients with other LQTS genotypes, particularly LQT2 and LQT3.10,11 The possibility that REM sleep represents a pro-arrhythmic state in patients with certain types of LQTS, as in the patient described here, remains an intriguing speculation.
Some reports are emerging about the utility of 24 h electrocardiographic monitoring of microvolt T-wave alternans as a marker of risk of ventricular arrythmias.12 In the future, this analysis could help to provide further insights into the etiology and mechanisms underlying nocturnal arrhythmogenesis. An additional advantage of 24 h electrocardiography is that it is possible to simultaneously monitor autonomic markers such as heart rate variability (autonomic tone) and heart rate turbulence (baroreceptor sensitivity). In another syndrome associated with nocturnal high-risk arrhythmias—Brugada syndrome—heart rate variability assessment reveals impaired autonomic function at night, which could predispose to the occurrence of ventricular fibrillation.13 This condition, therefore, demonstrates the potential for measuring these simultaneous parameters using 24 h electrocardiographic monitoring of T-wave alternans.
TREATMENT AND MANAGEMENT
Treatment options for the management of patients with ATS1 are limited. Although there is currently general agreement that LQT7 represents a disorder distinct from the classic Romano-Ward forms of LQTS (predominantly LQT1–3), similar treatment algorithms are often applied for these patients. Standard treatment for patients at risk includes prophylactic β-blockers. Patients refractory to β-blocker therapy can benefit from more complex therapies such as left-sided cardiac sympathetic denervation, pacemakers or implantable cardioverter-defibrillators, previously reviewed in this journal by Schwartz.14
In this case, significant ventricular ectopy persisted despite β-blocker therapy. Mexiletine has been reported previously for the treatment of muscular weakness15 and, therefore, had a potential role in this patient. As a class IB antiarrhythmic drug, mexiletine's primary mechanism of action is blocking the late sodium current of hNaV1.5 sodium channels. Consistently and reproducibly, the use of mexiletine was associated with a marked reduction in the patient's ventricular ectopy. Although the drug represents a rational therapeutic strategy in some patients with LQT3, it is unclear why it was so effective in reducing the severity of ventricular ectopy in this patient.
The fact that some patients with LQTS continue to have ectopic events despite β-blocker therapy, along with our theory that powerful bursts of parasympathetic activity occurring during REM sleep trigger ventricular instability, suggests that parasympatholytic drugs could be tested as an alternative therapy for preventing ventricular arrhythmias and SCD, both in patients with and without channelopathies.
CONCLUSIONS
In the present case study, ventricular ectopy during sleep was associated with the rare channelopathy, ATS1. The ectopy occurred in the absence of any sleep-related breathing disorder and was strongly associated with REM sleep. Intermittent fluctuations in cardiac sympathetic and parasympathetic tone during REM sleep might trigger ventricular arrhythmias, increasing vulnerability to sleep-related ventricular tachycardia and SCD. This hypothesis opens new lines of investigation for human SCD during sleep, which has been reported in apparently healthy, young Asian people, in some individuals with LQTS, and in patients with sleep apnea10,11 This theory could also explain why β-blockers alone might not prevent SCD, suggesting that parasympatholytic drugs could conceivably be an alternative therapy. This report also indicates a potential role for mexiletine in the management of patients with ATS1.
Acknowledgments
Dr Somers is supported by NIH grants HL-65176, HL-70302, HL-73211 and M01-RR00585, and Dr Ackerman's research program is supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program, the Dr Scholl Foundation, the CJ Foundation for SIDS, the Hannah Wernke Memorial Foundation, the AHA (Established Investigator Award), and the NIH (HD42569).
Footnotes
Vanderbilt Continuing Medical Education online This article offers the opportunity to earn one Category 1 credit toward the AMA Physician's Recognition Award.
Competing interests The authors declared they have no competing interests.
References
- 1.Sansone V, et al. Andersen's syndrome: a distinct periodic paralysis. Ann Neurol. 1997;42:305–312. doi: 10.1002/ana.410420306. [DOI] [PubMed] [Google Scholar]
- 2.Plaster NM, et al. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 3.Ai T, et al. Novel KCNJ2 mutation in familial periodic paralysis with ventricular dysrhythmia. Circulation. 2002;105:2592–2594. doi: 10.1161/01.cir.0000019906.35135.a3. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, et al. Electrocardiographic features in Andersen–Tawil syndrome patients with KCNJ2 mutations: characteristic T-U-wave patterns predict the KCNJ2 genotype. Circulation. 2005;111:2720–2726. doi: 10.1161/CIRCULATIONAHA.104.472498. [DOI] [PubMed] [Google Scholar]
- 5.Tsuboi M, Antzelevitch C. Cellular basis for electrocardiographic and arrhythmic manifestations of Andersen–Tawil syndrome (LQT7). Heart Rhythm. 2006;3:328–335. doi: 10.1016/j.hrthm.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackerman MJ. Cardiac channelopathies: it's in the genes. Nat Med. 2004;10:463–464. doi: 10.1038/nm0504-463. [DOI] [PubMed] [Google Scholar]
- 7.Somers VK, et al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 8.Verrier RL, et al. Sleep, dreams, and sudden death: the case for sleep as an autonomic stress test for the heart. Cardiovasc Res. 1996;31:181–211. [PubMed] [Google Scholar]
- 9.Manning JM, Cotten MD. Mechanism of cardiac arrhythmias induced by diencephalic stimulation. Am J Physiol. 1962;203:1120–1124. [Google Scholar]
- 10.Zareba W, et al. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960–965. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz PJ, et al. Genotype—phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 12.Verrier RL, et al. Noninvasive sudden death risk stratification by ambulatory ECG-based T-wave alternans analysis: evidence and methodological guidelines. Ann Noninvasive Electrocardiol. 10:110–120. doi: 10.1111/j.1542-474X.2005.10103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krittayaphong R, et al. Heart rate variability in patients with Brugada syndrome in Thailand. Eur Heart J. 2003;24:1771–1778. doi: 10.1016/j.ehj.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz PJ. Management of long QT syndrome. Nat Clin Pract Cardiovasc Med. 2005;2:346–351. doi: 10.1038/ncpcardio0239. [DOI] [PubMed] [Google Scholar]
- 15.Pouget J, Serratrice G. Myotonia with muscular weakness corrected by exercise: the therapeutic effect of mexiletine [French]. Rev Neurol (Paris) 1983;139:665–672. [PubMed] [Google Scholar]