Abstract
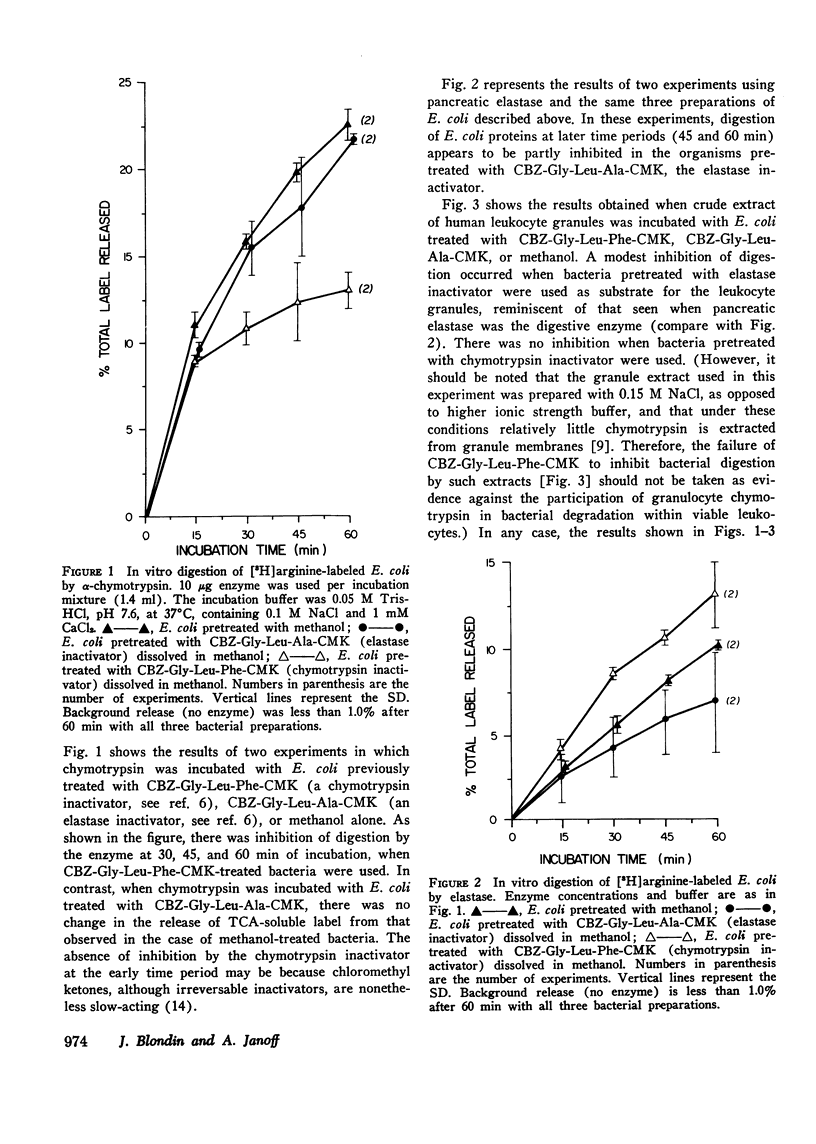
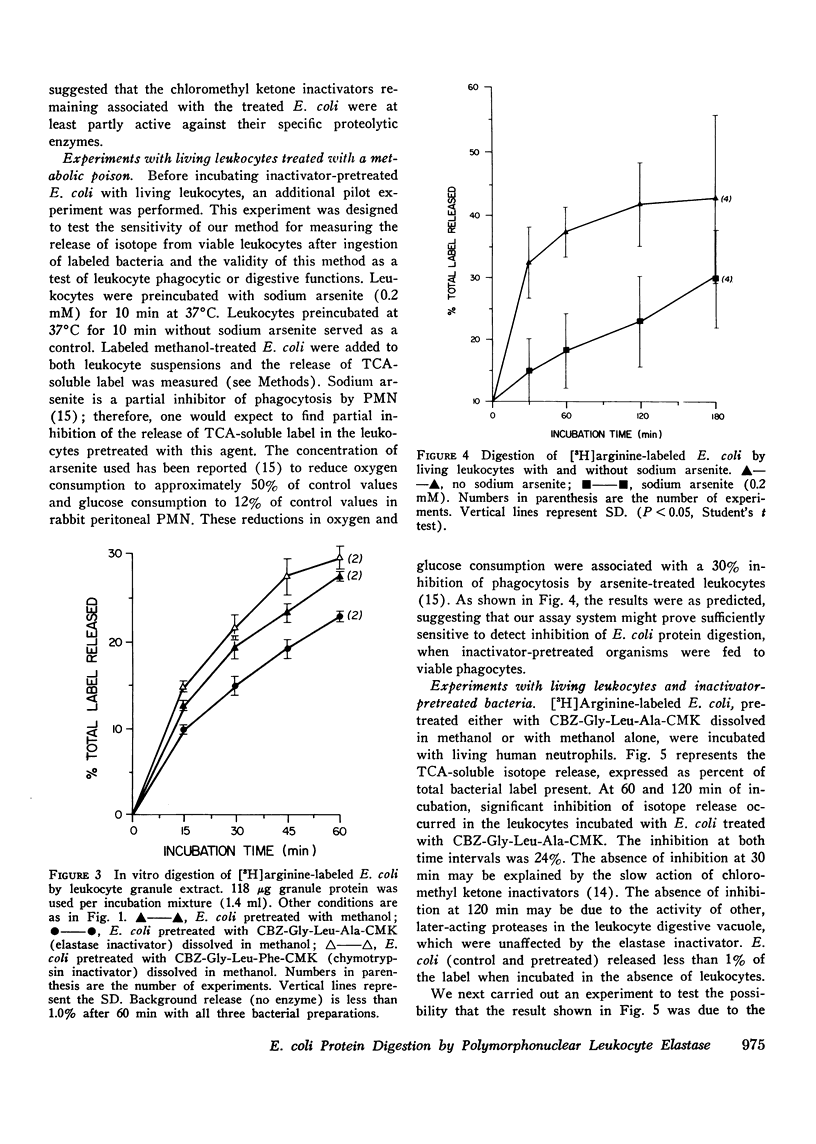
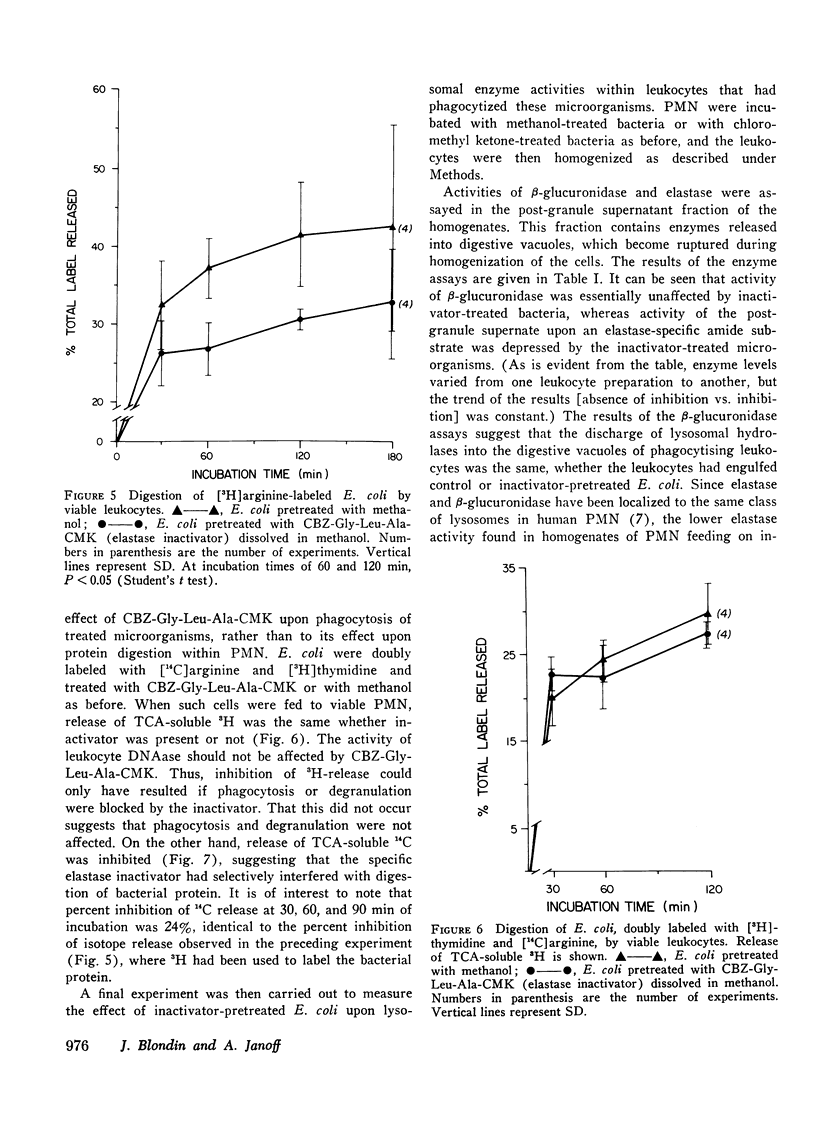
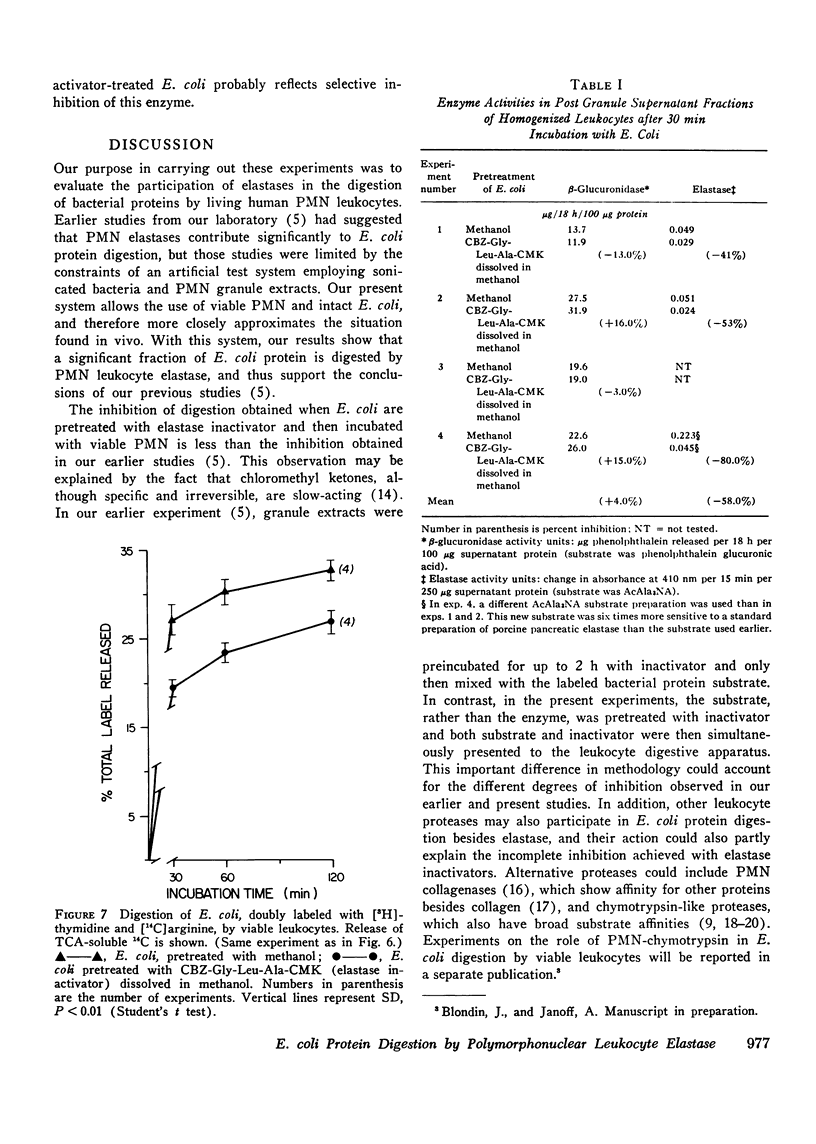
Human polymorphonuclear leukocyte (PMN) elastase has been implicated in various pathological conditions. However, its physiological role remains undefined. One possible function of this enzyme may be digestion of bacterial proteins after phagocytosis. To test this hypothesis, we prepared Escherichia coli labeled with [3H]arginine and treated these bacteria with a lipid-soluble, active-site-directed chloromethyl ketone inactivator of pancreatic and granulocyte elastases (carbobenzoxy-L-glycyl-L-leucyl-L-alanine chloromethyl ketone, dissolved in methanol). Control bacteria were treated with methanol alone. When E. coli pretreated with the inactivator were incubated with solutions of porcine pancreatic elastase or with PMN granule extract, release of trichloroacetic acid-soluble radioactivity was significantly lower than in the control E. coli. Similar results were obtained when treated and control E. coli were fed to viable human PMN. In contrast, release of trichloroacetic acid-soluble radioactivity from E. coli containing [3H]thymidine was not affected by pretreatment of bacteria with elastase inactivator before feeding them to PMN, suggesting that phagocytosis of E. coli had not been inhibited by the chloromethyl ketone. When treated and control bacteria were fed to PMN, no significant difference was observed in the activity of lysosomal beta-glucuronidase recovered from post-granule supernatant fractions of homogenized leukocytes, suggesting that lysosomal degranulation had not been suppressed by the inactivator. However, elastase activity of the same fractions was depressed if the leukocytes had phagocytized chloromethyl ketone-treated E. coli, suggesting that inhibition of PMN elastase had occurred. We conclude that PMN elastase participates in digestion of E. coli proteins by human PMN.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoub E. M., Wannamaker L. W. The fate of group A streptococci following phagocytosis. In vitro phagocytic studies of isotope-labeled streptococci. J Immunol. 1967 Dec;99(6):1099–1105. [PubMed] [Google Scholar]
- Brittinger G., Hirschhorn R., Douglas S. D., Weissmann G. Studies on lysosomes. XI. Characterization of a hydrolase-rich fraction from human lymphocytes. J Cell Biol. 1968 May;37(2):394–411. doi: 10.1083/jcb.37.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. I. Observations on the requirements and consequences of particle ingestion. J Exp Med. 1960 May 1;111:667–687. doi: 10.1084/jem.111.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Goldman J., Patriarca P. Phospholipid metabolism by phagocytic cells. VI. Observations on the fate of phospholipids of granulocytes and ingested Escherichia coli during phagocytosis. Biochim Biophys Acta. 1972 Sep 7;280(1):33–44. [PubMed] [Google Scholar]
- Feinstein G., Janoff A. A rapid method for purification of human granulocyte cationic neutral proteases: purification and characterization of human granulocyte chymotrypsin-like enzyme. Biochim Biophys Acta. 1975 Oct 22;403(2):477–492. doi: 10.1016/0005-2744(75)90076-5. [DOI] [PubMed] [Google Scholar]
- Feinstein G., Kupfer A., Sokolovsky M. N-acetyl-(L-Ala) 3 -p-nitroanilide as a new chromogenic substrate for elastase. Biochem Biophys Res Commun. 1973 Feb 20;50(4):1020–1026. doi: 10.1016/0006-291x(73)91508-8. [DOI] [PubMed] [Google Scholar]
- Galdston M., Janoff A., Davis A. L. Familial variation of leukocyte lysosomal protease and serum 1 -antitrypsin as determinants in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1973 May;107(5):718–727. doi: 10.1164/arrd.1973.107.5.718. [DOI] [PubMed] [Google Scholar]
- Gerber A. C., Carson J. H., Hadorn B. Partial purification and characterization of a chymotrypsin-like enzyme from human neutrophil leucocytes. Biochim Biophys Acta. 1974 Sep 11;364(1):103–112. doi: 10.1016/0005-2744(74)90137-5. [DOI] [PubMed] [Google Scholar]
- Janoff A., Blondin J. The effect of human granulocyte elastase on bacterial suspensions. Lab Invest. 1973 Oct;29(4):454–457. [PubMed] [Google Scholar]
- Janoff A., Blondin J. The role of elastase in the digestion of E. coli proteins by human polymorphonuclear leukocytes. I. Experiments in vitro. Proc Soc Exp Biol Med. 1974 Apr;145(4):1427–1430. doi: 10.3181/00379727-145-38027. [DOI] [PubMed] [Google Scholar]
- Janoff A., Feinstein G., Malemud C. J., Elias J. M. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. I. Penetration of enzyme into rabbit articular cartilage and release of 35SO4-labeled material from the tissue. J Clin Invest. 1976 Mar;57(3):615–624. doi: 10.1172/JCI108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968 Nov 1;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser H., Greenwald R. A., Feinstein G., Janoff A. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. II. Degradation of isolated bovine nasal cartilage proteoglycan. J Clin Invest. 1976 Mar;57(3):625–632. doi: 10.1172/JCI108318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Brown R. S., Bladen H. A., Fullmer H. M. Degradation of collagen by a human granulocyte collagenolytic system. J Clin Invest. 1968 Dec;47(12):2622–2629. doi: 10.1172/JCI105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of two granulocyte collagenases. Eur J Biochem. 1973 Jul 16;36(2):473–481. doi: 10.1111/j.1432-1033.1973.tb02932.x. [DOI] [PubMed] [Google Scholar]
- Rindler-Ludwig R., Braunsteiner H. Cationic proteins from human neutrophil granulocytes. Evidence for their chymotrypsin-like properties. Biochim Biophys Acta. 1975 Feb 27;379(2):606–617. doi: 10.1016/0005-2795(75)90167-1. [DOI] [PubMed] [Google Scholar]
- STAHELIN H., KARNOVSKY M. L., SUTER E. Studies on the interaction between phagocytes and tubercle bacilli. II. The action of phagocytes upon C14-labelled tubercle bacilli. J Exp Med. 1956 Jul 1;104(1):137–150. doi: 10.1084/jem.104.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Isolation of elastase-like and chymotrypsin-like neutral proteases from human granulocytes. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1077–1082. doi: 10.1515/bchm2.1974.355.2.1077. [DOI] [PubMed] [Google Scholar]
- Shaw E. Selective chemical modification of proteins. Physiol Rev. 1970 Apr;50(2):244–296. doi: 10.1152/physrev.1970.50.2.244. [DOI] [PubMed] [Google Scholar]
- Tuhy P. M., Powers J. C. Inhibition of human leukocyte elastase by peptide chloromethyl ketones. FEBS Lett. 1975 Feb 15;50(3):359–361. doi: 10.1016/0014-5793(75)80527-8. [DOI] [PubMed] [Google Scholar]



