Abstract
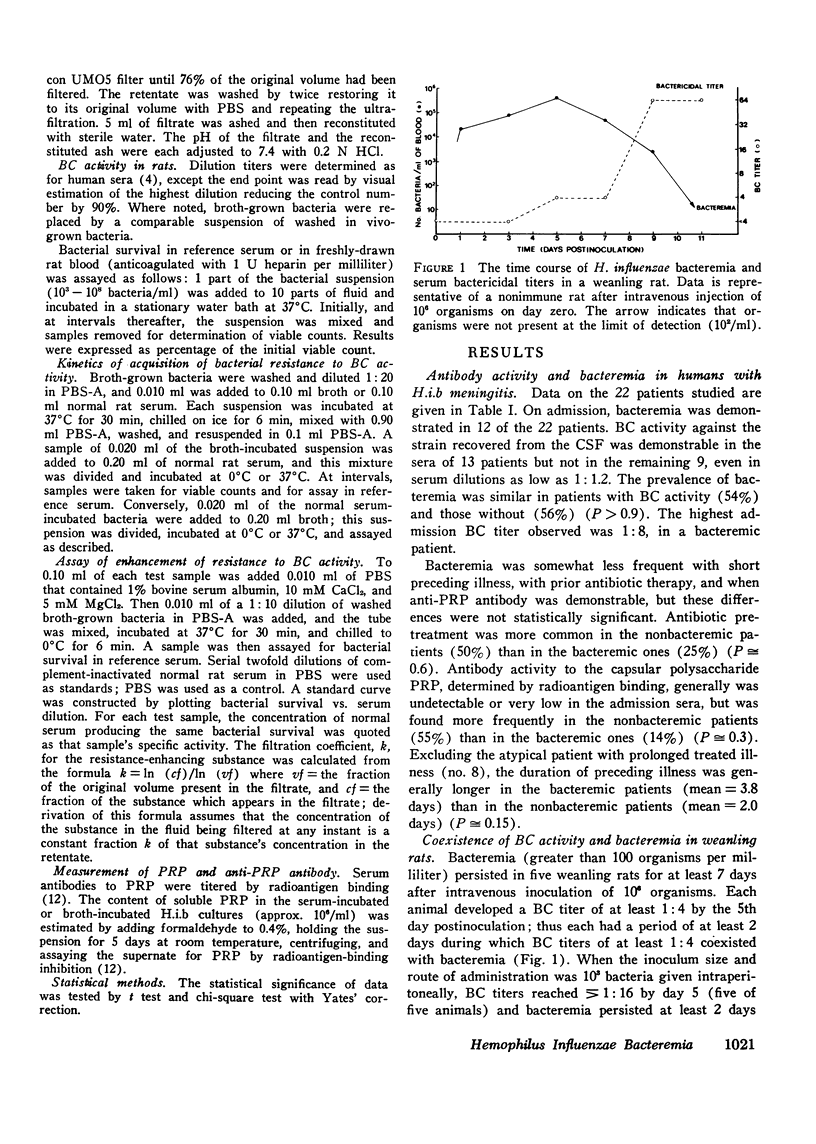
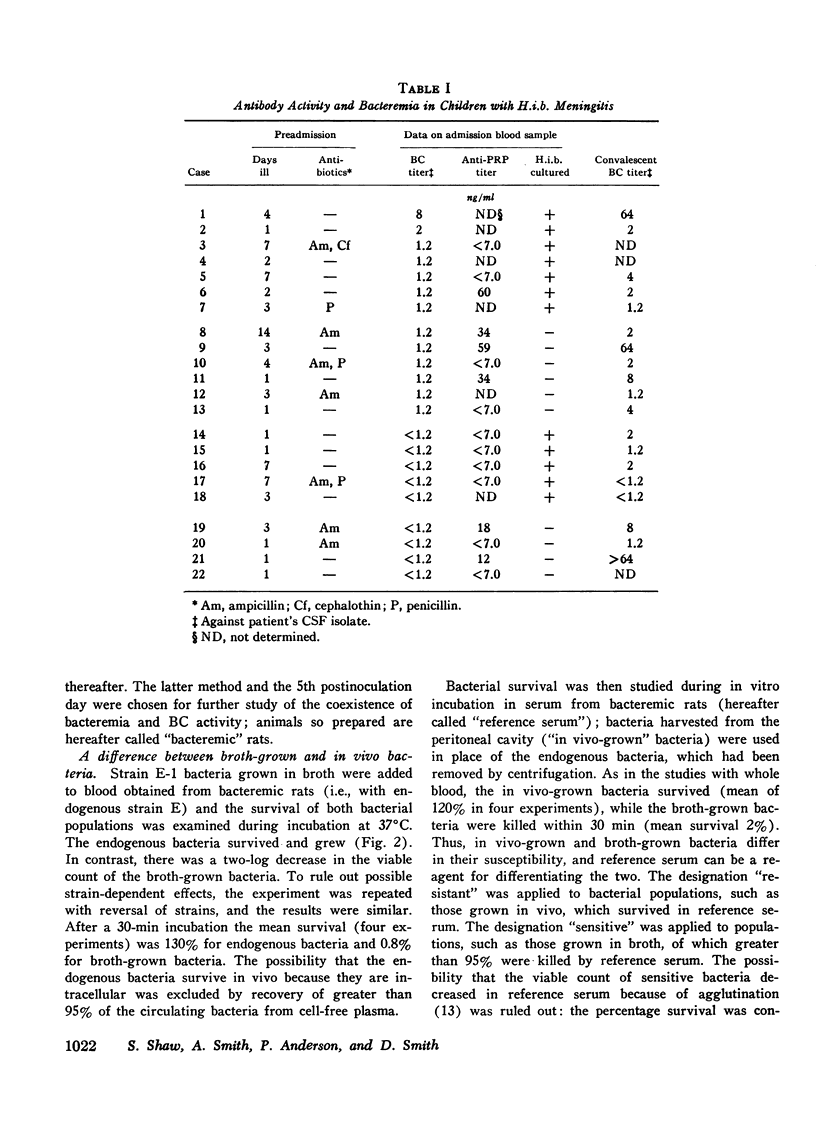
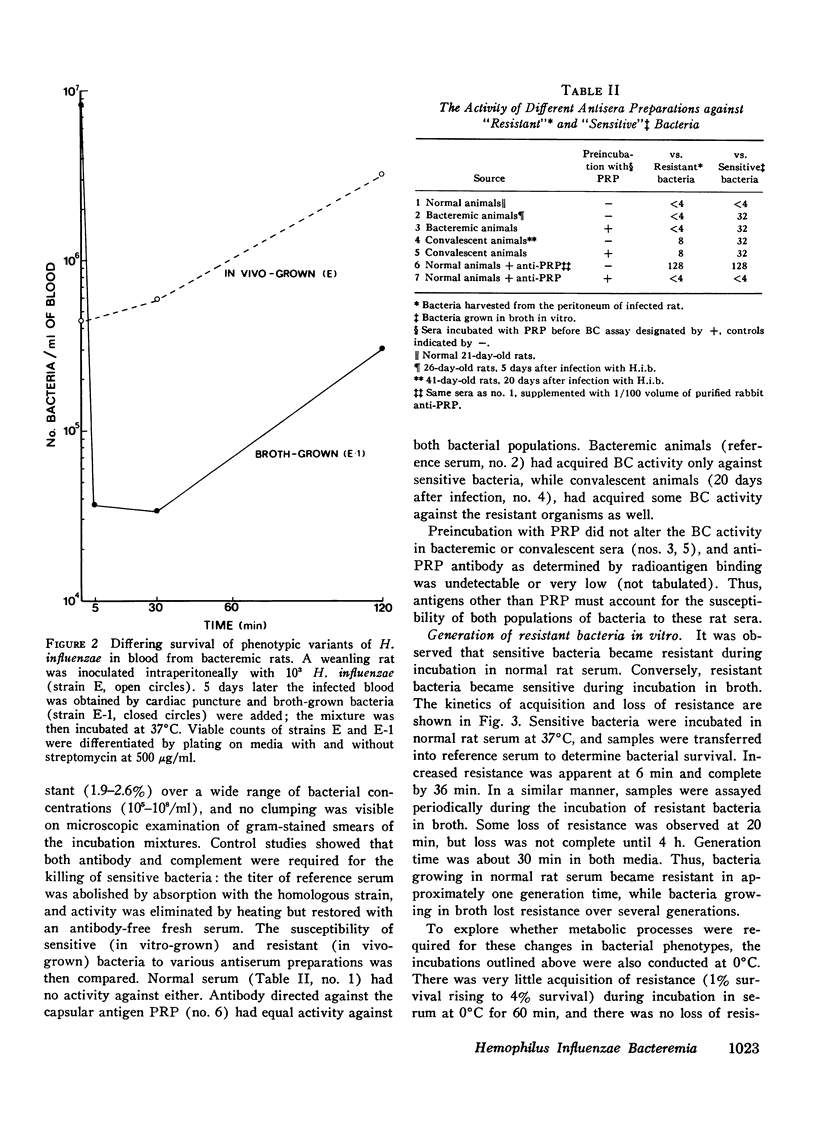
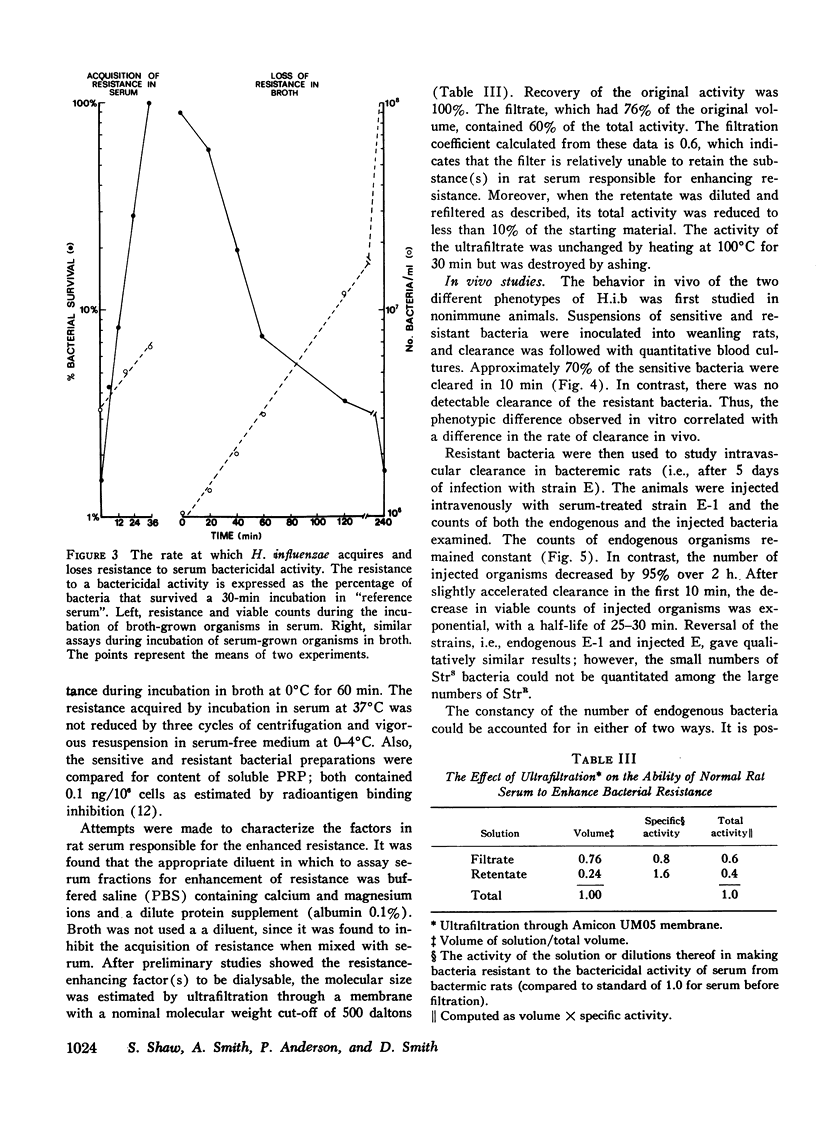
We investigated the role of serum bactericidal activity in Hemophiplus influenzae type b infections in infants with meningitis and in a rat model. In infected infants, 13/22 admission sera had bactericidal activity against the infecting strain, and bacteremia was as frequent in those with bactericidal activity (54%) as those without (56%). The coexistence of bactericidal activity and bacteremia was reproduced and studied in experimentally infected weanling rats. Serum from such rats kills in vitro 95% of conventionally broth-grown bacteria within 10 min, but does not kill organisms obtained from the infected animals. Thus bactericidal activity as conventionally determined for H. influenzae b may have no relevance in vivo, Incubation of broth-grown bacteria in normal rat serum for 30 min at 37 degrees C produces a resistance like that of in vivo organisms. This phenotypic conversion depends on factors that are of molecular weight less than 1,000, stable to 100 degrees C, but destroyed by ashing. When injected intravenously into nonimmune animals, broth-grown bacteria are quickly cleared, while serum-preincubated bacteria are not. The latter, however, are cleared when injected into bacteremic rats (half-life 30 min). Bacteremia in the rats may persist despite this capacity for clearance because bacteria are entering the blood from extravascular fluids, which contain greater than 90% of the total bacterial burden.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIN R. V. Mechanism of immunity in haemorrhagic septicaemia. Nature. 1960 May 28;186:734–735. doi: 10.1038/186734b0. [DOI] [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Boyer F. A proposed mechanism for natural immunity to enterobacterial pathogens. J Immunol. 1968 Feb;100(2):292–306. [PubMed] [Google Scholar]
- DAVIS S. D., WEDGWOOD R. J. KINETICS OF THE BACTERICIDAL ACTION OF NORMAL SERUM ON GRAM-NEGATIVE BACTERIA. J Immunol. 1965 Jul;95:75–79. [PubMed] [Google Scholar]
- Feigin R. D., Richmond D., Hosler D. W., Shackelford P. G. Reassessment of the role of bactericidal antibody in Hemophilus influenzae infection. Am J Med Sci. 1971 Dec;262(6):338–346. doi: 10.1097/00000441-197112000-00005. [DOI] [PubMed] [Google Scholar]
- Feingold D. S. The serum bactericidal reaction. IV. Phenotypic conversion of Escherichia coli from serum-resistance to serum-sensitivity by diphenylamine. J Infect Dis. 1969 Oct;120(4):437–444. doi: 10.1093/infdis/120.4.437. [DOI] [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump D. W., Tarr P., Phillips C. A., Forsyth B. R. Bactericidal antibodies to Hemophilus influenzae. Proc Soc Exp Biol Med. 1971 Oct;138(1):76–80. doi: 10.3181/00379727-138-35835. [DOI] [PubMed] [Google Scholar]
- Hall W. H., Manion R. E., Zinneman H. H. Blocking serum lysis of Brucella abortus by hyperimmune rabbit immunoglubulin A. J Immunol. 1971 Jul;107(1):41–46. [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melching L., Vas S. I. Effects of serum components on gram-negative bacteria during bactericidal reactions. Infect Immun. 1971 Jan;3(1):107–115. doi: 10.1128/iai.3.1.107-115.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melching L., Vas S. I. The effects of serum components on the agglutination of Gram-negative bacteria. Can J Microbiol. 1970 Feb;16(2):121–124. doi: 10.1139/m70-020. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Waldo B., Johnston R. B., Jr Separation of serum bactericidal and opsonizing activities for Haemophilus influenzae type b. Infect Immun. 1973 Sep;8(3):488–490. doi: 10.1128/iai.8.3.488-490.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden C. W., Melish M., Overall J. C., Jr, Baum J. Immunologic responses to Hemophilus influenzae meningitis. J Pediatr. 1972 Feb;80(2):209–214. doi: 10.1016/s0022-3476(72)80580-8. [DOI] [PubMed] [Google Scholar]
- Norden C. W., Michaels R. Immunologic response in patients with epiglottitis caused by Haemophilus influenzae type b. J Infect Dis. 1973 Dec;128(6):777–780. doi: 10.1093/infdis/128.6.777. [DOI] [PubMed] [Google Scholar]
- Norden C. W. Prevalence of bactericidal antibodies to Haemophilus influenzae, type b. J Infect Dis. 1974 Nov;130(5):489–494. doi: 10.1093/infdis/130.5.489. [DOI] [PubMed] [Google Scholar]
- O'Reilly R. J., Anderson P., Ingram D. L., Peter G., Smith D. H. Circulating polyribophosphate in Hemophilus influenzae, type b meningitis. Correlation with clinical course and antibody response. J Clin Invest. 1975 Oct;56(4):1012–1022. doi: 10.1172/JCI108148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROANTREE R. J., PAPPAS N. C. The survival of strains of enteric bacilli in the blood stream as related to their sensitivity to the bactericidal effect of serum. J Clin Invest. 1960 Jan;39:82–88. doi: 10.1172/JCI104031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Rowley D. Sensitization of complement resistant bacterial strains. Nature. 1969 Mar 29;221(5187):1259–1261. doi: 10.1038/2211259a0. [DOI] [PubMed] [Google Scholar]
- Rowley D. Sensitivity of rough gram-negative bacteria to the bactericidal action of serum. J Bacteriol. 1968 May;95(5):1647–1650. doi: 10.1128/jb.95.5.1647-1650.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D., Turner K. J. Passive sensitization of Salmonella adelaide to the bactericidal action of antibody and complement. Nature. 1968 Feb 17;217(5129):657–658. doi: 10.1038/217657a0. [DOI] [PubMed] [Google Scholar]
- SMITH H., FITZGEORGE R. B. THE CHEMICAL BASIS OF THE VIRULENCE OF BRUCELLA ABORTUS. V. THE BASIS OF INTRACELLULAR SURVIVAL AND GROWTH IN BOVINE PHAGOCYTES. Br J Exp Pathol. 1964 Apr;45:174–186. [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B. Induction of serum Haemophilus influenzae type B capsular antibodies in adult volunteers fed cross-reacting Escherichia coli 075:K100:H5. N Engl J Med. 1975 May 22;292(21):1093–1096. doi: 10.1056/NEJM197505222922103. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H., Averill D. R., Jr, Marino J., Moxon E. R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973 Aug;8(2):278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Peter G., Ingram D. L., Harding A. L., Anderson P. Responses of children immunized with the capsular polysaccharide of Hemophilus influenzae, type b. Pediatrics. 1973 Nov;52(5):637–644. [PubMed] [Google Scholar]
- WARDLAW A. C. The complement-dependent bacteriolytic activity of normal human serum. I. The effect of pH and ionic strength and the role of lysozyme. J Exp Med. 1962 Jun 1;115:1231–1249. doi: 10.1084/jem.115.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. B., Jr Phagocytosis, with particular reference to encapsulated bacteria. Bacteriol Rev. 1960 Mar;24(1):41–49. doi: 10.1128/br.24.1.41-49.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisbren B. A., Brown I. A factor in the serum of patients with persisting infection that inhibits the bactericidal activity of normal serum against the organism that is causing the infection. J Immunol. 1966 Sep;97(3):431–437. [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Glynn A. A. Gonococci in urethral exudates possess a virulence factor lost on subculture. Nature. 1970 Jul 25;227(5256):382–384. doi: 10.1038/227382a0. [DOI] [PubMed] [Google Scholar]



