Abstract
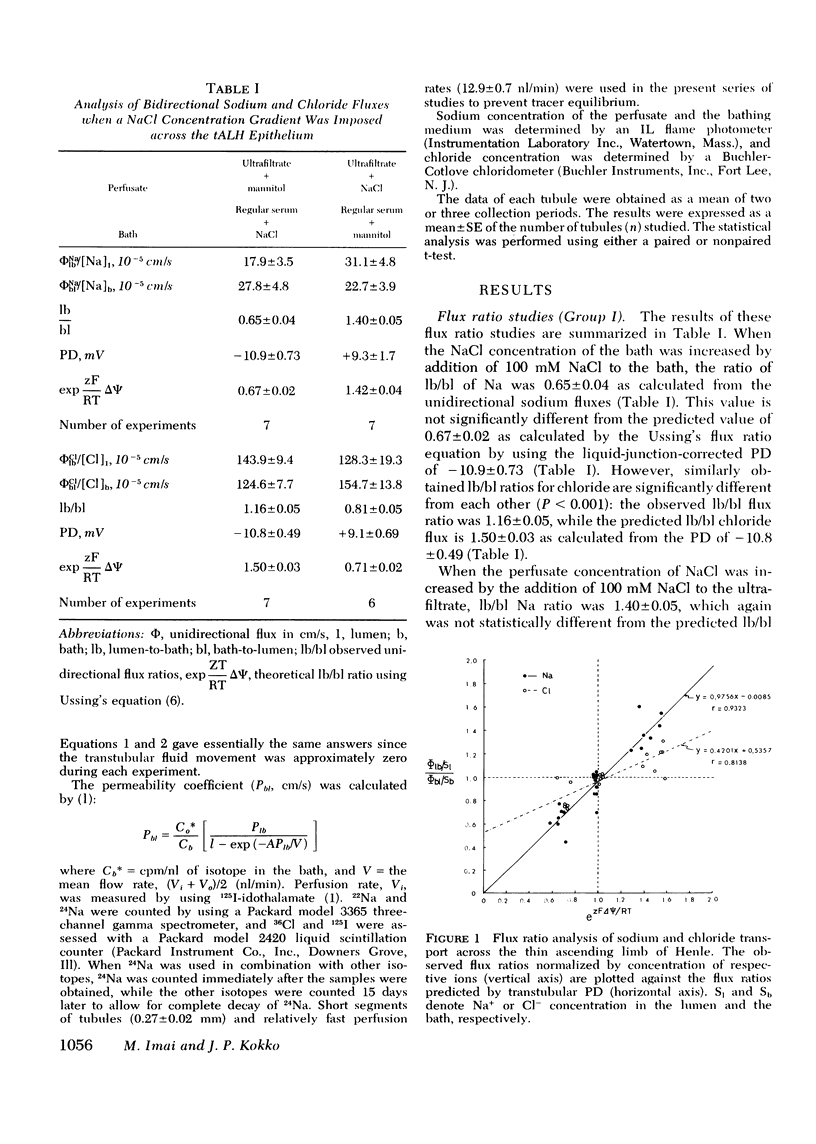
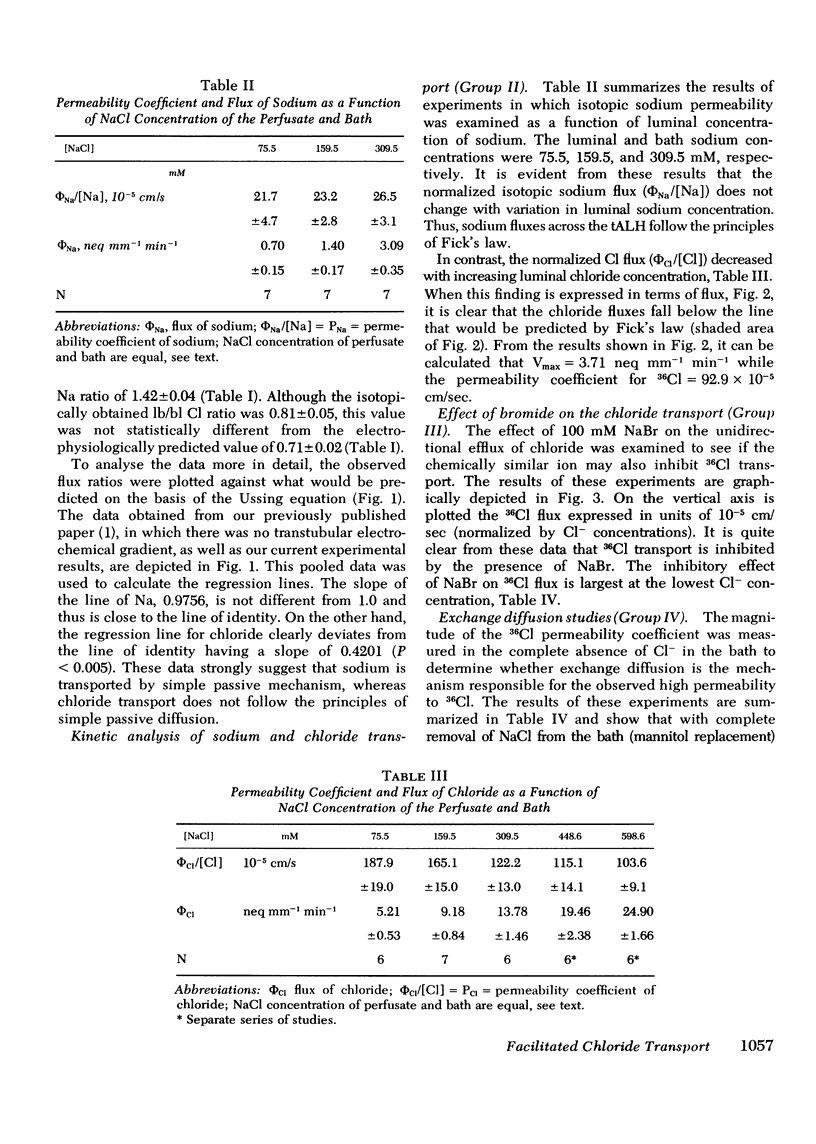
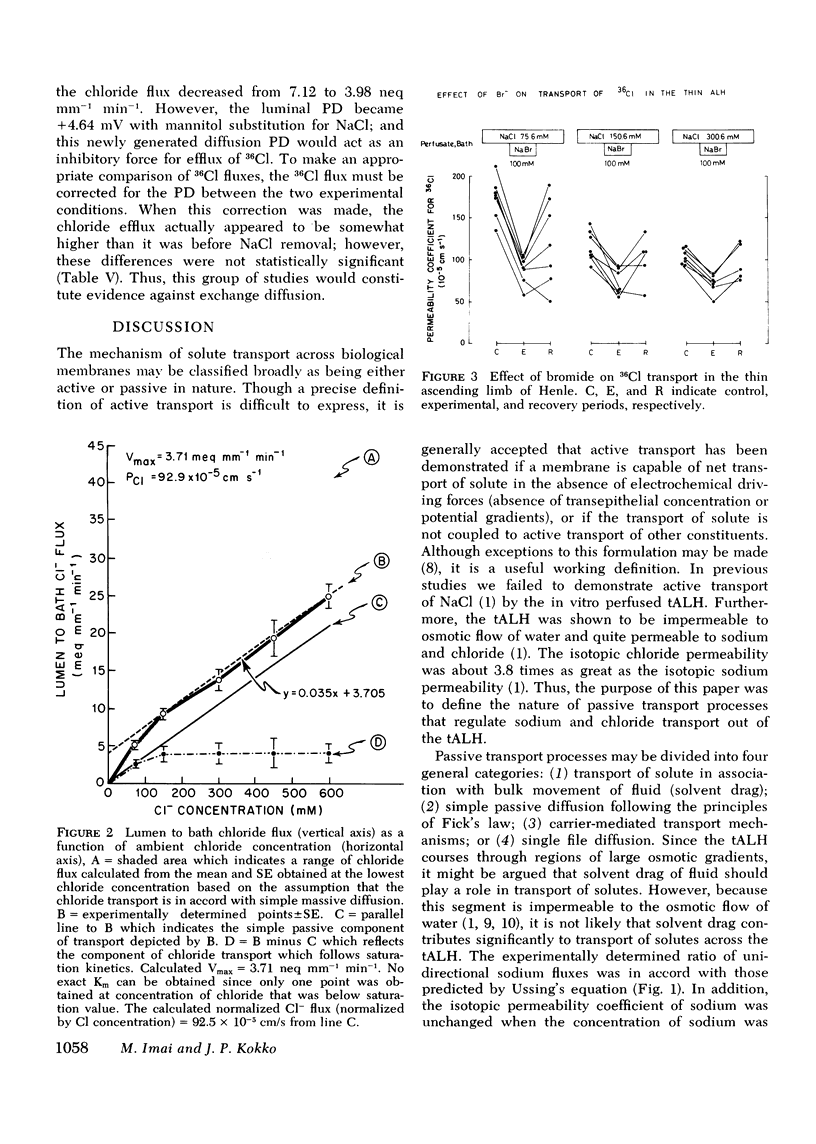
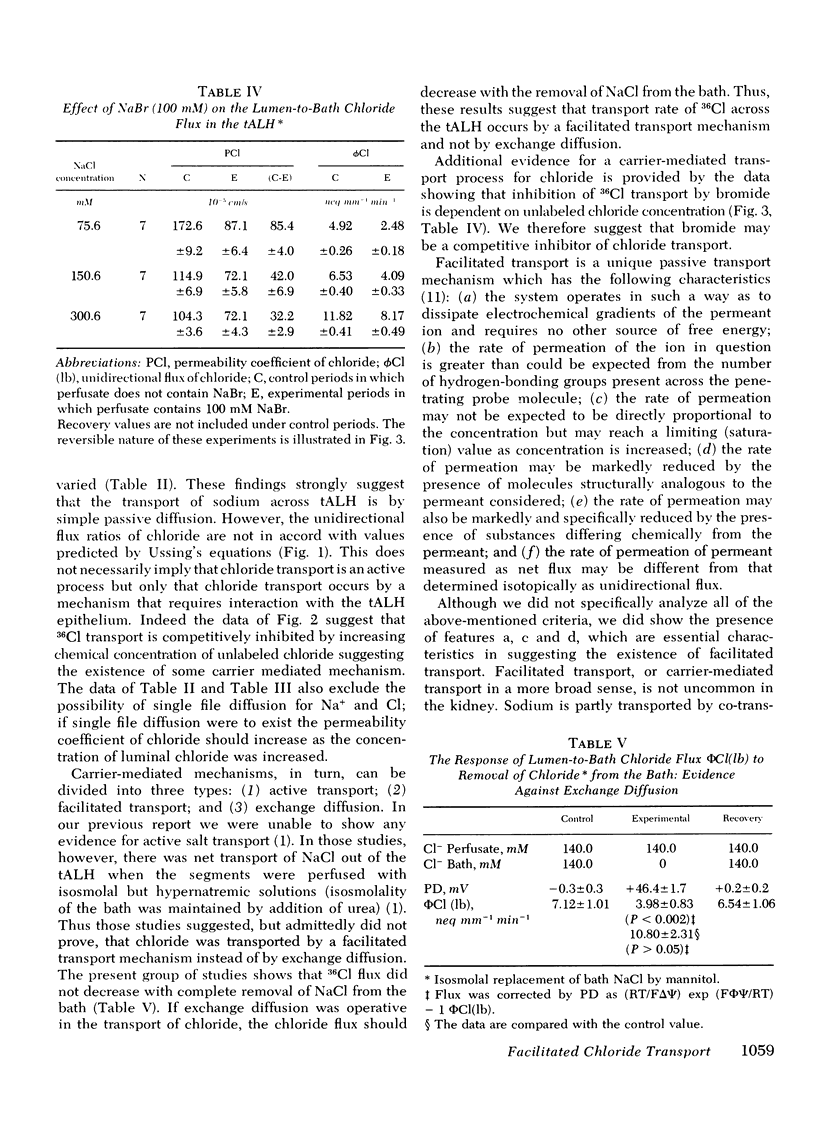
Our previous in vitro studies have disclosed that the thin ascending limb of Henle (tALH) possesses some unique membrane characteristics. In those studies we failed to demonstrated active transport of sodium chloride by the tALH, although it was shown that the isotopic permeability to sodium and chloride was unusually high. However, we did not examine the mechanisms by which the apparent high permeation of sodium chloride occurs. Thus the purpose of the present studies was to elucidate the mechanism of sodium chloride transport across the isolated tALH of the rabbit by conducting four different types of studies: (1) comparison of the observed chloride and sodium flux ratios to those predicted by Ussing's equation under imposed salt concentration gradients; (2) kinetic evaluation of chloride and sodium fluxes; (3) examination of the effect of bromide on the kinetics of chloride transport; and (4) experiments to test for the existence of exchange diffusion of chloride. In the first set of studies the predicted and the theoretical flux ratios of sodium were identical in those experiments in which sodium chloride was added either to the perfusate or to the bath. However, the observed chloride flux ratio, lumen-to-bath/bath-to-lumen, was significantly lower than that predicted from Ussing's equation when 100 mM sodium chloride was added to the bath. In the second set of experiments the apparent isotopic permeability for sodium and for chloride was measured under varying perfusate and bath NaCl concentrations. There was no statistical change in the apparent sodium permeability coefficient when the NaCl concentration was raised by varying increments from 85.5 to 309.5 mM. However, permeation of 36Cl decrease significantly with an increase in Cl from 73.6 to 598.6 mM. These events could be explained by a two component chloride transport process consisting of simple diffusion and a saturable facilitated diffusion process with a Vmax = 3.71 neq mm-1 min-1. In the third set of studies it was shown that bromide inhibits transport of chloride and that the magnitude of inhibition is dependent on chloride concentrations. The fourth set of studies ruled out the existence of exchange diffusion. In conclusion, these studies indicate that sodium transport across tALH is by simple passive diffusion, while chloride transport across tALH involves at least two mechanisms: (1) simple passive diffusion; and (2) a specific membrane interaction process (carrier-mediated) which is competitively inhibited by bromide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bresler E. H. On criteria for active transport. J Theor Biol. 1967 Jul;16(1):135–146. doi: 10.1016/0022-5193(67)90057-4. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973 Mar;224(3):659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- Dantzler W. H. Characteristics of urate transport by isolated perfused snake proximal renal tubules. Am J Physiol. 1973 Feb;224(2):445–453. doi: 10.1152/ajplegacy.1973.224.2.445. [DOI] [PubMed] [Google Scholar]
- FOX M., THIER S., ROSENBERG L., SEGAL S. IONIC REQUIREMENTS FOR AMINO ACID TRANSPORT IN THE RAT KIDNEY CORTEX SLICE. I. INFLUENCE OF EXTRACELLULAR IONS. Biochim Biophys Acta. 1964 Jan 27;79:167–176. doi: 10.1016/0926-6577(64)90049-x. [DOI] [PubMed] [Google Scholar]
- GORESKY C. A., WATANABE H., JOHNS D. G. THE RENAL EXCRETION OF FOLIC ACID. J Clin Invest. 1963 Dec;42:1841–1849. doi: 10.1172/JCI104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Hillman R. E., Albrecht I., Rosenberg L. E. Transport of amino acids by isolated rabbit renal tubules. Biochim Biophys Acta. 1968 Apr 29;150(3):528–530. doi: 10.1016/0005-2736(68)90155-7. [DOI] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Sodium chloride, urea, and water transport in the thin ascending limb of Henle. Generation of osmotic gradients by passive diffusion of solutes. J Clin Invest. 1974 Feb;53(2):393–402. doi: 10.1172/JCI107572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P. Proximal tubule potential difference. Dependence on glucose on glucose, HCO 3 , and amino acids. J Clin Invest. 1973 Jun;52(6):1362–1367. doi: 10.1172/JCI107308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P., Rector F. C. Flow dependence of transtubular potential difference in isolated perfused segments of rabbit proximal convoluted tubule. J Clin Invest. 1971 Dec;50(12):2745–2750. doi: 10.1172/JCI106776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P., Rector F. C., Jr Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 1972 Oct;2(4):214–223. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- Kokko J. P. Sodium chloride and water transport in the descending limb of Henle. J Clin Invest. 1970 Oct;49(10):1838–1846. doi: 10.1172/JCI106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P. Urea transport in proximal tubule and the descending limb of Henle. J Clin Invest. 1972 Aug;51(8):1999–2008. doi: 10.1172/JCI107006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. Permeability of the loop of Henle, vasa recta, and collecting duct to water, urea, and sodium. Am J Physiol. 1968 Jul;215(1):108–115. doi: 10.1152/ajplegacy.1968.215.1.108. [DOI] [PubMed] [Google Scholar]
- ROSENBERG L. E., BLAIR A., SEGAL S. Transport of amino acids by slices of rat-kidney cortex. Biochim Biophys Acta. 1961 Dec 23;54:479–488. doi: 10.1016/0006-3002(61)90088-9. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedas G., Weiss C. Die Wirkung von Anderungen der Natriumkonzentration im Perfusionsmedium und von Strophanthin auf die Glucoseresorption der isolierten Rattenniere. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;298(1):12–22. [PubMed] [Google Scholar]
- Scriver C. R., Mohyuddin F. Amino acid transport in kidney. Heterogeneity of alpha-aminoisobutyric uptake. J Biol Chem. 1968 Jun 25;243(12):3207–3213. [PubMed] [Google Scholar]
- VOGEL G., LAUTERBACH F., KROEGER W. DIE BEDEUTUNG DES NATRIUMS FUER DIE RENALEN TRANSPORTE VON GLUCOSE UND PARA-AMINOHIPPURSAEURE. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Mar 18;283:151–159. [PubMed] [Google Scholar]
- Vogel G., Tervooren U., Stoeckert I. Untersuchungen zur Abhängigkeit des renal tubulären Glucose-Transportes vom Ionen-Angebot sowie des Na+-Transportes vom Angebot an Glucose. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;288(4):359–368. [PubMed] [Google Scholar]



