Abstract
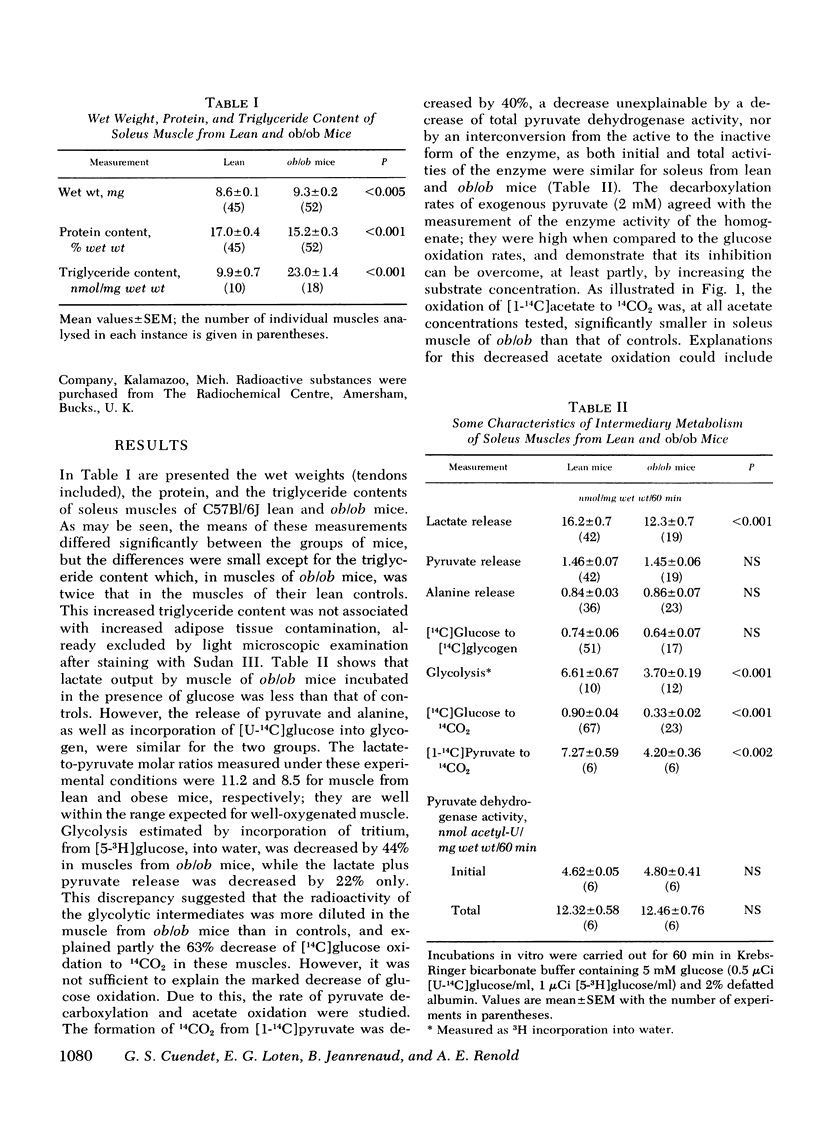
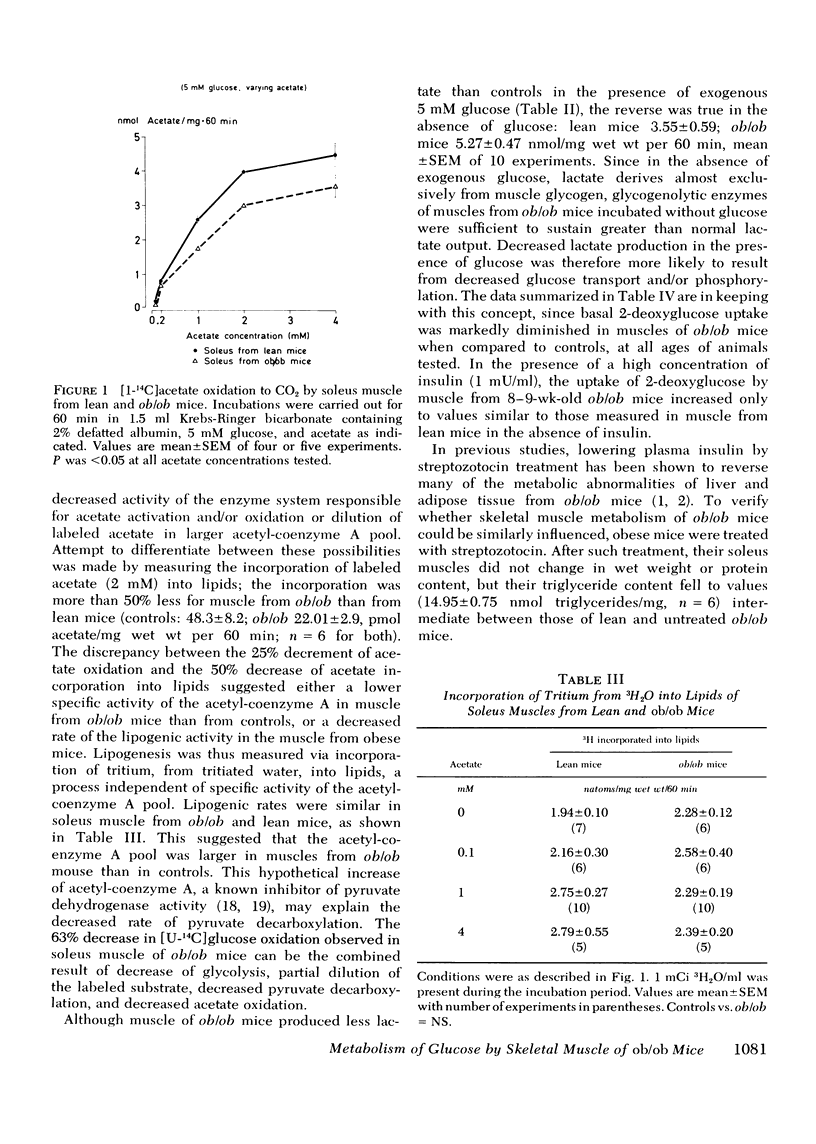
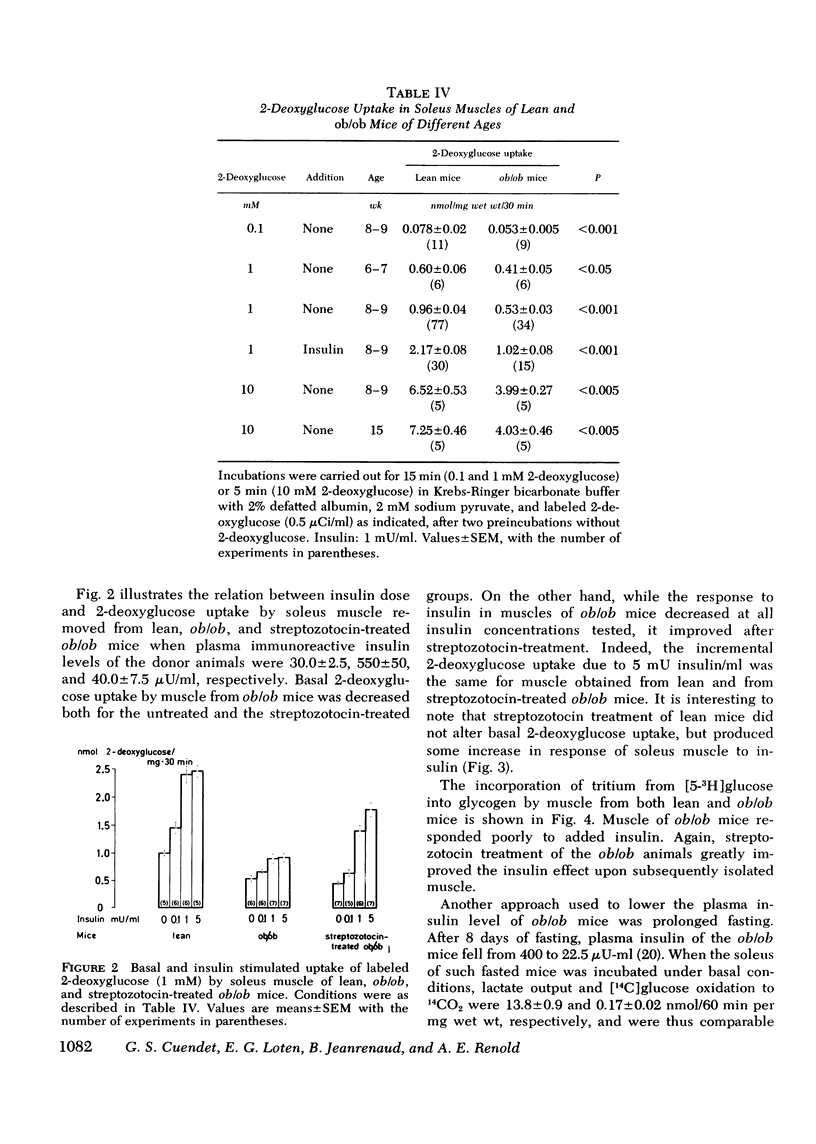
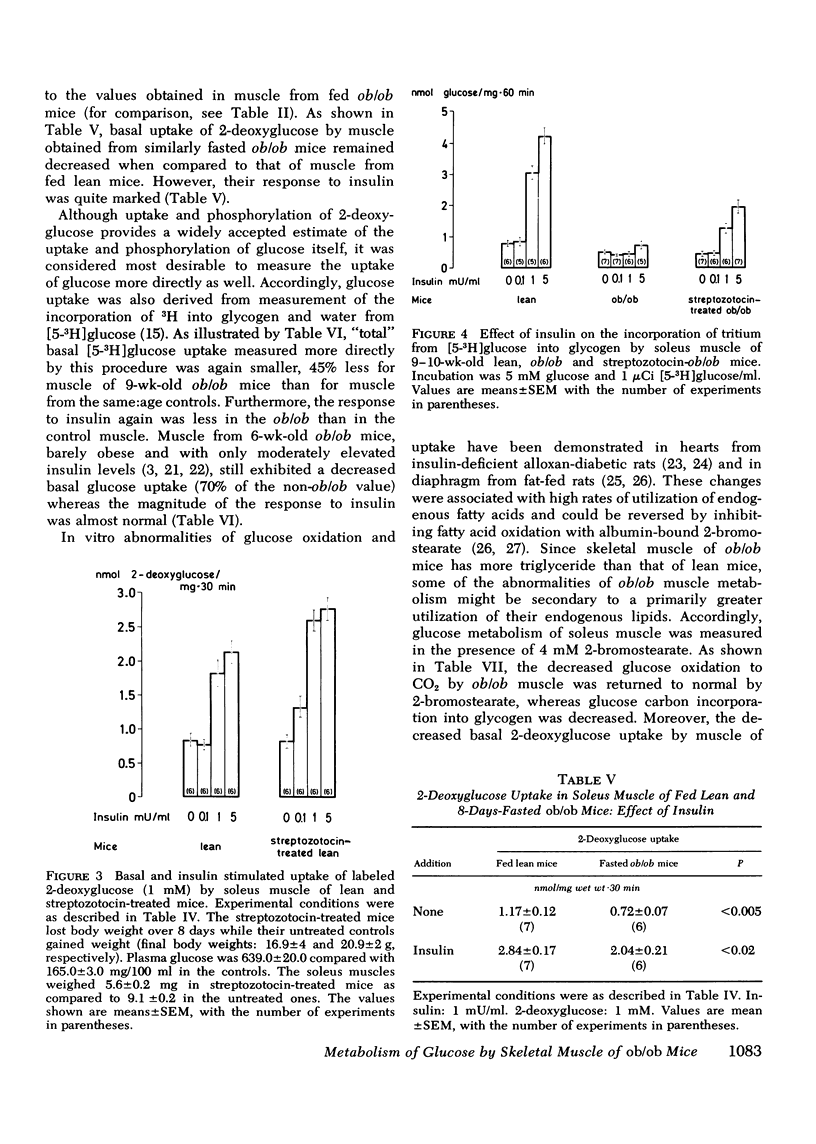
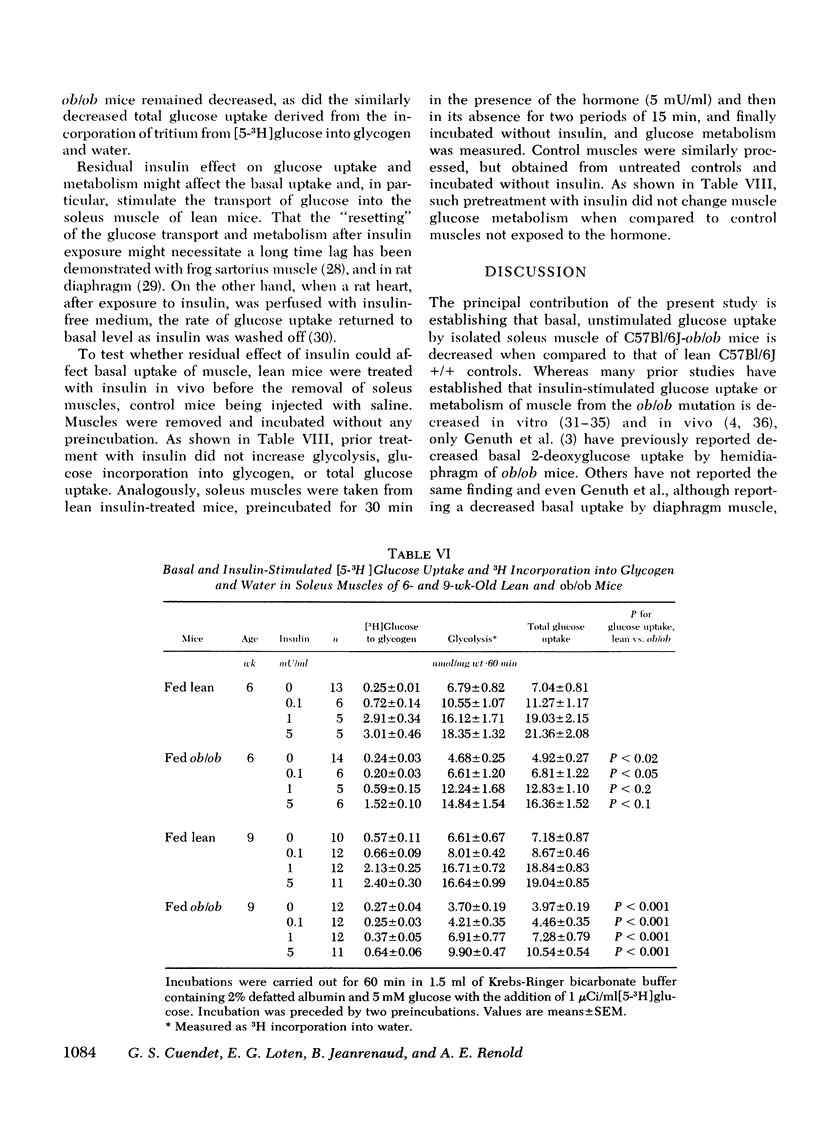
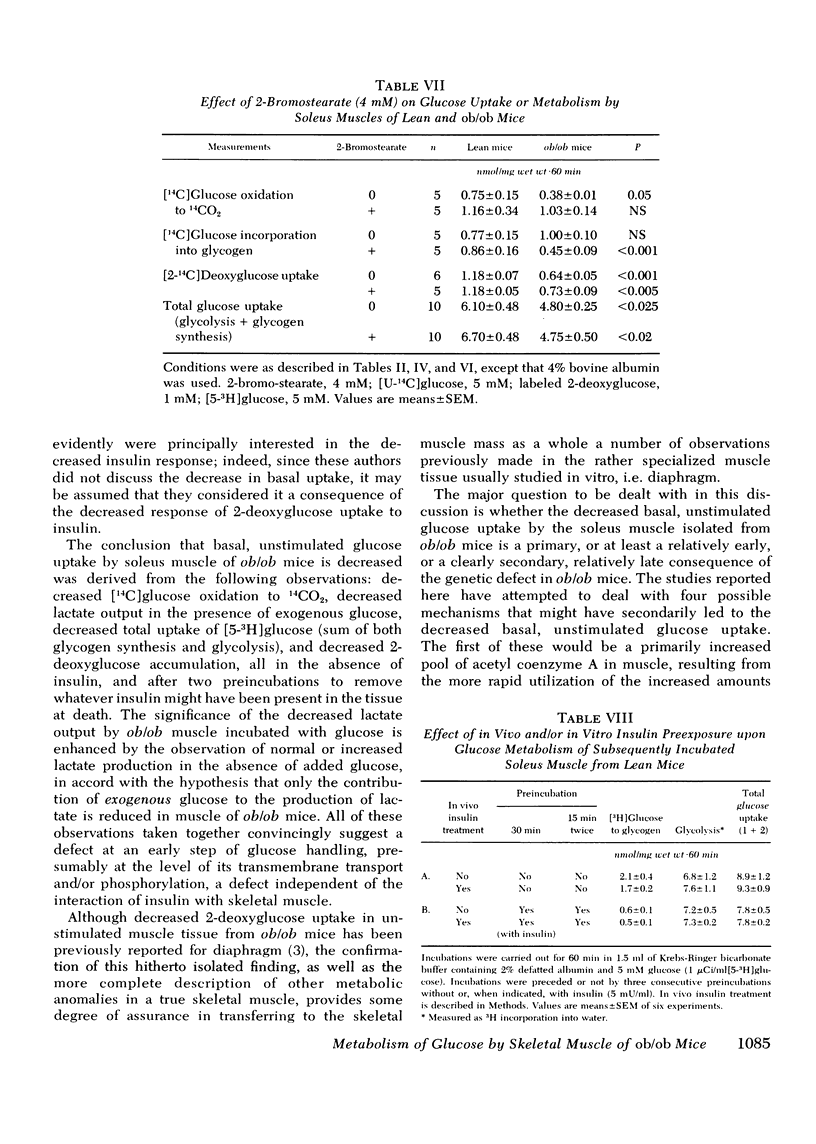
Insulin resistance of diaphragms of ob/ob mice has been repeatedly demonstrated previously both in vitro and in vivo. In the present study, transport and metabolism of glucose with and without insulin stimulation were compared in a skeletal muscle more likely than diaphragm or heart to be representative of the overall striated muscle mass, i.e. isolated soleus muscle. Compared with soleus muscle from lean controls, unstimulated lactate release in the presence of exogenous glucose was depressed from 16.2 to 12.3 nmol/60 min per mg wet wt in soleus from ob/ob mutants; glycolysis was decreased from 6.6 to 3.7 and [14C]glucose oxidation to 14CO2 from 0.90 to 0.33 nmol glucose/60 min per mg wet wt. Uptake of 2-deoxyglucose (2-DOG), both with and without insulin, was very much less for soleus from ob/ob than from lean mice, at 2-DOG concentrations ranging from 0.1 to 10 mM, and in mice of 6-15 wk. When 2-DOG concentration was 1 mM, its basal uptake was 0.53 nmol/30 min per mg wet wt for soleus of ob/ob as against 0.96 for soleus of lean mice. The absolute increment due to 1 mU/ml insulin was 0.49 in muscle of ob/ob as against 1.21 in that of lean mice. When the resistance to insulin action was decreased by pretreatment in vivo by either streptozotocin injection or fasting, the decreased basal 2-DOG uptake of subsequently isolated soleus muscle was not improved. Inhibition of endogenous oxidation of fatty acids by 2-bromostearate, while greatly increasing 14CO2 production from [14C]glucose, did not affect basal [5-3H]glucose metabolism or 2-DOG uptake. It is suggested that transport and/or phosphorylation of glucose under basal, unstimulated conditions are depressed in soleus muscle of ob/ob mice, whether or not resistance to insulin and hyperinsulinemia are also present. Although the origin of the decreased basal glucose uptake remains unknown it might be related to a similar decrease in basal glucose uptake by ventromedial hypothalamic cells, an event presumably resulting in a tendency to hyperphagia. Decreased basal glucose uptake by soleus muscle of ob/ob mice might explain the hyperglycemia, and hence partly the hyperinsulinemia and excessive fat deposition of those animals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R. R., Beloff-Chain A. Hormonal control of intermediary metabolism in obese hyperglycemic mice. I. The sensitivity and response to insulin in adipose tissue and muscle in vitro. Diabetes. 1971 Aug;20(8):522–534. doi: 10.2337/diab.20.8.522. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Bassett J. M., Randle P. J. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972 Feb;126(3):525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F., Singh A., Le Marchand Y., Loten E. G., Jeanrenaud B. Abnormalities in lipogenesis and triglyceride secretion by perfused livers of obese-hyperglycaemic (ob-ob) mice: relationship with hyperinsulinaemia. Diabetologia. 1974 Apr;10(2):155–162. doi: 10.1007/BF01219673. [DOI] [PubMed] [Google Scholar]
- Baile C. A., Herrera M. G., Mayer J. Ventromedial hypothalamus and hyperphagia in hyperglycemic obese mice. Am J Physiol. 1970 Mar;218(3):857–863. doi: 10.1152/ajplegacy.1970.218.3.857. [DOI] [PubMed] [Google Scholar]
- Batchelor B. R., Stern J. S., Johnson P. R., Mahler R. J. Effects of streptozotocin on glucose metabolism, insulin response, and adiposity in ob/ob mice. Metabolism. 1975 Jan;24(1):77–91. doi: 10.1016/0026-0495(75)90009-8. [DOI] [PubMed] [Google Scholar]
- Batt R., Mialhe P. Insulin resistance of the inherently obese mouse--obob. Nature. 1966 Oct 15;212(5059):289–290. doi: 10.1038/212289a0. [DOI] [PubMed] [Google Scholar]
- Bringolf M., Zaragoza N., Rivier D., Felber J. P. Studies on the metabolic effects induced in the rat by a high-fat diet. Inhibition of pyruvate metabolism in diaphragm in vitro and its relation to the oxidation of fatty acids. Eur J Biochem. 1972 Apr 11;26(3):360–367. doi: 10.1111/j.1432-1033.1972.tb01775.x. [DOI] [PubMed] [Google Scholar]
- Chaudry I. H., Gould M. K. Kinetics of glucose uptake in isolated soleus muscle. Biochim Biophys Acta. 1969 May 6;177(3):527–536. doi: 10.1016/0304-4165(69)90315-8. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Coleman D. L. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973 Aug;9(4):294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuendet G. S., Loten E. G., Cameron D. P., Renold A. E., Marliss E. B. Hormone-substrate responses to total fasting in lean and obese mice. Am J Physiol. 1975 Jan;228(1):276–283. doi: 10.1152/ajplegacy.1975.228.1.276. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Debons A. F., Krimsky I., From A., Cloutier R. J. Rapid effects of insulin on the hypothalamic satiety center. Am J Physiol. 1969 Oct;217(4):1114–1118. doi: 10.1152/ajplegacy.1969.217.4.1114. [DOI] [PubMed] [Google Scholar]
- Debons A. F., Krimsky I., Likuski H. J., From A., Cloutier R. J. Gold thioglucose damage to the satiety center: inhibition in diabetes. Am J Physiol. 1968 Mar;214(3):652–658. doi: 10.1152/ajplegacy.1968.214.3.652. [DOI] [PubMed] [Google Scholar]
- Freychet P., Laudat M. H., Laudat P., Rosselin G., Kahn C. R., Gorden P., Roth J. Impairment of insulin binding to the fat cell plasma membrane in the obese hyperglycemic mouse. FEBS Lett. 1972 Sep 15;25(2):339–342. doi: 10.1016/0014-5793(72)80519-2. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Randle P. J. Regulation of glucose uptake by muscles. 10. Effects of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, and of fatty acids, ketone bodies and pyruvate, on the glycerol output and concentrations of free fatty acids, long-chain fatty acyl-coenzyme A, glycerol phosphate and citrate-cycle intermediates in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):678–687. doi: 10.1042/bj0930678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genuth S. M., Przybylski R. J., Rosenberg D. M. Insulin resistance in genetically obese, hyperglycemic mice. Endocrinology. 1971 May;88(5):1230–1238. doi: 10.1210/endo-88-5-1230. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. VI. The penetration and phosphorylation of 2-deoxyglucose in the diaphram of diabetic rats. J Biol Chem. 1960 Nov;235:3070–3075. [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Roth J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J Biol Chem. 1973 Jan 10;248(1):244–250. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Marchand Y., Singh A., Assimacopoulos-Jeannet F., Orci L., Rouiller C., Jeanrenaud B. A role for the microtubular system in the release of very low density lipoproteins by perfused mouse livers. J Biol Chem. 1973 Oct 10;248(19):6862–6870. [PubMed] [Google Scholar]
- Loten E. G., Rabinovitch A., Jeanrenaud B. In vivo studies on lipogenesis in obese hyperglycaemic (ob-ob) mice: possible role of hyperinsulinaemia. Diabetologia. 1974 Feb;10(1):45–52. doi: 10.1007/BF00421413. [DOI] [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P. Studies of tissue permeability. IX. The effect of insulin on the penetration of 3-methylglucose-H3 in frog muscle. J Biol Chem. 1963 Jan;238:40–49. [PubMed] [Google Scholar]
- Panksepp J. Hypothalamic regulation of energy balance and feeding behavior. Fed Proc. 1974 May;33(5):1150–1165. [PubMed] [Google Scholar]
- Stauffacher W., Lambert A. E., Vecchio D., Renold A. E. Measurements of insulin activities in pancreas and serum of mice with spontaneous ("Obese" and "New Zealand Obese") and induced (Goldthioglucose) obesity and hyperglycemia, with considerations on the pathogenesis of the spontaneous syndrome. Diabetologia. 1967 Apr;3(2):230–237. doi: 10.1007/BF01222200. [DOI] [PubMed] [Google Scholar]
- Stauffacher W., Renold A. E. Effect of insulin in vivo on diaphragm and adipose tissue of obese mice. Am J Physiol. 1969 Jan;216(1):98–105. doi: 10.1152/ajplegacy.1969.216.1.98. [DOI] [PubMed] [Google Scholar]
- Westman S. Development of the obese-hyperglycaemic syndrome in mice. Diabetologia. 1968 Jun;4(3):141–149. doi: 10.1007/BF01219435. [DOI] [PubMed] [Google Scholar]



