Ten or twenty years ago, glial cells were considered minor players in the nervous system, even though they outnumber neurons 10-fold. Glia were thought to function as passive support cells, bringing nutrients to and removing wastes from the neurons, whereas the latter carried out the critical nervous system functions of information processing, plasticity, learning, and memory. Recent studies, reviewed here, are changing this view and demonstrating that glial cells play a key role in these essential brain functions [Sharma and Vijayaraghavan (1) and Ullian et al. (2)].
A diversity of glial cell types is expressed in the central nervous system (CNS), each with distinct functions. Oligodendrocytes form myelin, the tight wrappings of lipid-rich membrane layers that coat nerve cell axons, serving as electrical insulation that speeds up the propagation of action potentials down the long nerve cell process. Astrocytes ensheath synapses, the specialized intercellular contact sites that function in the rapid transfer of information from neurons to their target cells. The function of astrocytes is largely undefined. Many reports show that astrocytes express ion channels, both ligand-gated and voltage-dependent (3), and are proposed to have the general function of clearing neurotransmitters and ions away from the synapse (4). However, recent groundbreaking studies of the identity and function of ion channels on astrocytes suggest that these cells have a more direct and active role in synapse function.
One such study by Sharma and Vijayaraghavan (1) shows that astrocytes share many excitable properties with neurons, but there are also some interesting differences. This report shows that hippocampal astrocytes in vitro express functional neuronal nicotinic acetylcholine receptors (nAChRs). The nAChRs are the highly Ca2+ permeable α7 subtype that are widely expressed in the vertebrate brain (5). Sharma and Vijayaraghavan (1) demonstrate that activation of these receptors produces rapid excitatory inward currents and an increase in intracellular Ca2+ in the astrocytes. Interestingly, the study shows two differences between α7-nAChRs on astrocytes and neurons. First, the density of the functional receptors is an order of magnitude lower on astrocytes. Second, different calcium signaling mechanisms are used by the receptors on the two cell types. In astrocytes, α7-nAChR activation induces a rapid and large increase in intracellular Ca2+ levels by receptor-mediated Ca2+ influx that is amplified by the entering Ca2+ triggering Ca2+ release from caffeine-sensitive internal stores. In contrast, the increase in internal Ca2+ concentration in neurons upon α7-nAChR activation is largely because of influx through voltage-gated Ca2+ channels. Blocking the voltage-gated Ca2+ channels has no effect on Ca2+ influx in astrocytes (1). As α7-nAChR current density is relatively low on astrocytes and these receptors desensitize rapidly, the functional coupling of receptor activation to Ca2+-induced Ca2+-release channels may be critical to achieve physiologically significant changes in intracellular Ca2+ levels in these nonneuronal cells. As the Sharma and Vijayaraghavan study focuses on astrocytes in vitro where nAChRs are activated by bath-applied ligand, it is important to show that in vivo astrocytes respond to ACh. It will be especially interesting to establish the physiological conditions that induce nAChR activation on astrocytes as well as nAChR levels and spatial distribution in vivo, particularly in relation to the synapse, because the presynaptic terminal is a potential endogenous source of the neurotransmitter that activates receptors on nonneuronal cells. It is intriguing to consider that nAChRs on astrocytes may contribute to the wide-ranging cholinergic receptor functions in the brain. These functions include excitatory synaptic transmission between specific neurons, modulating neurotransmitter release from presynaptic terminals, reinforcing nicotine addiction, and increasing attention, arousal, and short-term memory formation (6, 7).
The key question is: What is the functional significance of receptor activation and increases in intracellular Ca2+ levels in these nonneuronal cells? Recent innovative studies, including one by Parpura and Haydon (8), have shown that activation of functional glutamate receptors on astrocytes produces an increase in intracellular Ca2+ levels and the Ca2+-dependent release of glutamate, the major excitatory neurotransmitter in the vertebrate CNS (9) (see Fig. 1). The glial-released glutamate activates neighboring neurons in culture. In this way, astrocytes enhance interneuronal synaptic transmission. Such astrocyte responses can be initiated by neurotransmitter released from the neuronal presynaptic terminal (10). Altogether, these studies demonstrate that there is activity-dependent rapid intercellular signaling between astrocytes and neurons (at least in vitro) and that this signaling modulates interneuronal synaptic activity. The work of Sharma and Vijayaraghavan extends this field by adding nAChRs to the repertoire of astrocyte receptors that increase intracellular Ca2+. In fact, many receptor types are expressed on astrocytes, including opioid, dopamine, GABAA, glycine, serotonin, β-adrenergic, and purinergic receptors (11). Important recent studies further show that astrocyte–neuron intercellular signaling likely occurs in situ. In the acutely isolated retina and hippocampal slice, activation of astrocytes elevates internal Ca2+ levels, resulting in transmitter release and the modulation of electrical activity in the adjacent neurons (12–15). Overall, these studies suggest that astrocyte–neuron intercellular signaling is widespread throughout the CNS and that astrocytes play an important active role in modulating synaptic communication between neurons.
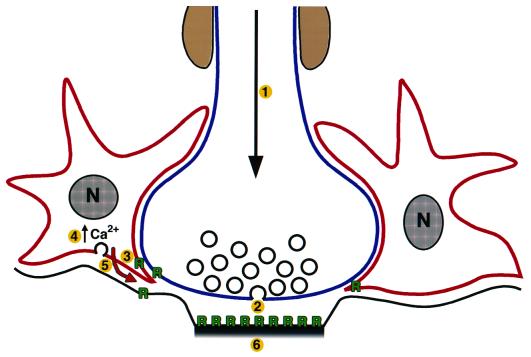
Figure 1.
Schematic of astrocyte–neuron intercellular signaling. Action potential firing in the neuron (1) induces neurotransmitter release from the presynaptic terminal (2), receptor activation on adjacent astrocytes (3), increases in intracellular Ca2+ (4), Ca2+-dependent neurotransmitter release from the astrocytes (5), and the activation of neighboring neurons and potentiation of interneuronal synaptic transmission (6). In addition, astrocyte-derived soluble neurotrophic factors (red arrow) are also likely to effect CNS synapse function. R, neurotransmitter receptor.
The new study by Ullian, Barres, and colleagues (2) demonstrates another exciting unexpected glial cell function—astrocytes profoundly increase synapse number and are required for their maintenance in vitro. This effect is likely related, at least in part, to the capability of astrocytes to increase synaptic activity, as described above. Earlier attempts to determine the effects of glia on central synapses were hindered because most CNS neurons require glia for survival in vitro. To circumvent this experimental obstacle, the Barres lab developed a means of maintaining a purified pool of rodent retinal ganglion cells in culture. By adding glia back to the cultures, Pfrieger and Barres (16) demonstrated for the first time the surprising result that astrocytes increase the number and efficacy of CNS neuron synapses. The new study (2) extends these results by showing that astrocytes increase by 7-fold the number of synapses on each neuron and enhance synaptic efficacy by altering both presynaptic and postsynaptic functions. Interestingly, the numerous indices used to assess pre- and postsynaptic functions (including whole-cell patch-clamp recording, quantal analyses, and FM1–43 imaging of synaptic vesicle recycling) all showed quantitatively similar 7-fold increases. The astrocyte-induced increases in synapse formation and function are not because of increased neuron survival or maturation. Instead, the data suggest that there is an astrocyte-dependent reorganization of existing pre- and postsynaptic proteins to produce new synapses. Moreover, the neurons require the continued presence of the astrocytes for synapse maintenance, as glial removal causes a 4-fold reduction in synapse number. Altogether, the Barres lab studies define a novel and important function for astrocytes to induce and stabilize CNS synapses.
Determining whether astrocytes play a similar role in vivo and identifying the molecular mechanisms that underlie the astrocyte-induced alterations in neurons are important issues to resolve. Preliminary studies from the Barres lab have already begun to address such questions. Their results show that during normal development, most retinal ganglion cells have extended their axons into the superior colliculus target field by embryonic day 16, but their synapse formation is largely delayed until astrocytes appear, around postnatal day 7 (2). Direct astrocyte–neuron contact is likely not required for the potentiating effects of astrocytes, because medium “conditioned” by astrocytes in culture is capable of promoting synapse formation by retinal ganglion cells (2, 16). Soluble neurotrophic factors have previously been shown to enhance pre- and postsynaptic differentiation, synaptic efficacy, and ion channel function (17, 18). Although preliminary attempts to identify specific glial-derived neurotrophic factors responsible for the action of astrocytes on ganglion cell synapses have yet to strike gold (16), the involvement of such a signaling molecule is considered likely (see Fig. 1). In addition, the excitable properties of astrocytes may also contribute to their effects on synapse number and function. Astrocytes respond to and release neurotransmitter rapidly and cause increases in neuron excitation and synaptic transmission, as described above. Precedence shows that increased synaptic activity can cause long-term increases in synapse number and strength, forming the basis of the Hebbian model of the synaptic plasticity that underlies learning and memory (19). Thus, multiple astrocyte-derived signals are likely to mediate the enhancement of CNS synapse formation and function.
The field of glial cell biology is advancing rapidly. The innovative work reviewed here demonstrates unexpected functions of glia that go well beyond the “supporting role” in which they were cast historically. It is now clear that detailed knowledge of the conversation between astrocytes and neurons will be essential for understanding the development of the nervous system and its response during learning, struggles with injury, and changes with aging.
Acknowledgments
We thank Brian Williams for preparation of the figure and Dr. Kathleen Dunlap for critical reading of the manuscript. Work in our laboratory is supported by National Institutes of Health Grant NS 21725.
Footnotes
See companion article on page 4148.
References
- 1.Sharma G, Vijayaraghavan S. Proc Natl Acad Sci USA. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. . (First Published March 20, 2001; 10.1073/pnas.071540198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ullian E M, Sapperstein S K, Christopherson K S, Barres B A. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 3.Porter J T, McCarthy K D. Prog Neurobiol. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 4.Henn F A, Hamberger A. Proc Natl Acad Sci USA. 1971;68:2686–2690. doi: 10.1073/pnas.68.11.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Role L W, Berg D K. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 6.Lena C, Changeux J P. Curr Opin Neurobiol. 1997;7:674–682. doi: 10.1016/s0959-4388(97)80088-8. [DOI] [PubMed] [Google Scholar]
- 7.MacDermott A B, Role L W, Siegelbaum S A. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 8.Parpura V, Haydon P G. Proc Natl Acad Sci USA. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araque A, Parpura V, Sanzgiri R P, Haydon P G. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 10.Dani J W, Smith S J. Ciba Found Symp. 1995;188:195–205. doi: 10.1002/9780470514696.ch11. [DOI] [PubMed] [Google Scholar]
- 11.Verkhratsky A, Steinhauser C. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- 12.Pasti L, Volterra A, Pozzan T, Carmignoto G. J Neurosci. 1999;15:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini B L, Pozzan T, Volterra A. Nature (London) 1998;15:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 14.Kang J, Jiang L, Goldman S A, Nedergaard M. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 15.Newman E A, Zahs K R. J Neurosci. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfrieger F W, Barres B A. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 17.Vicario-Abejon C, Collin C, McKay R D, Segal M. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blondel O, Collin C, McCarran W J, Zhu S, Zamostiano R, Gozes I, Brenneman D E, McKay R D. J Neurosci. 2000;20:8012–8020. doi: 10.1523/JNEUROSCI.20-21-08012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hebb D O. The Organization of Behaviour. New York: Wiley; 1949. [Google Scholar]