Abstract
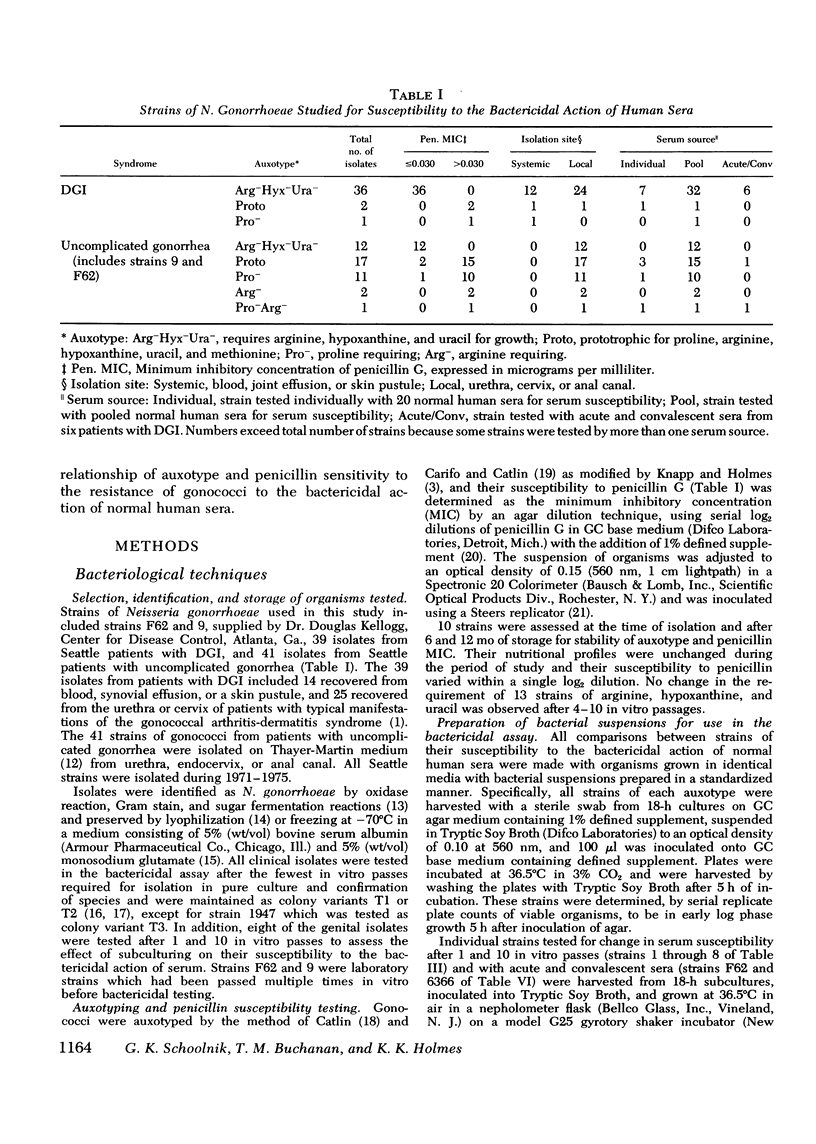
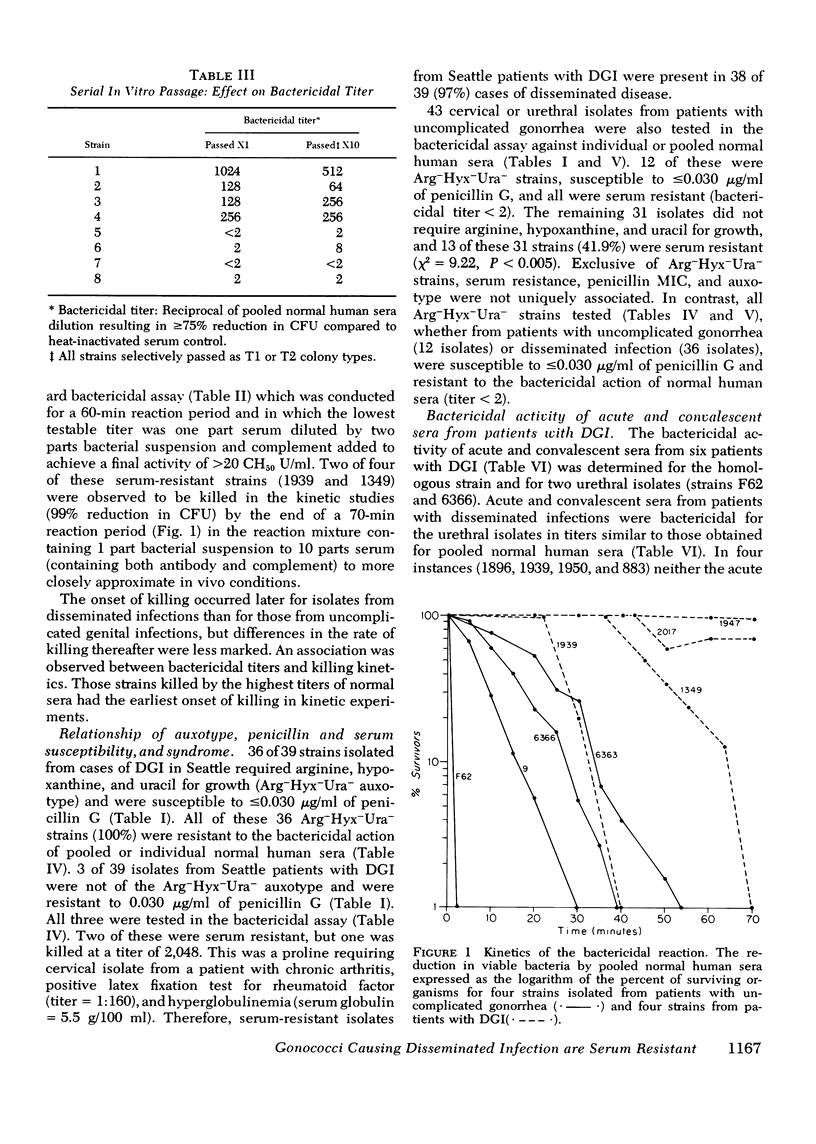
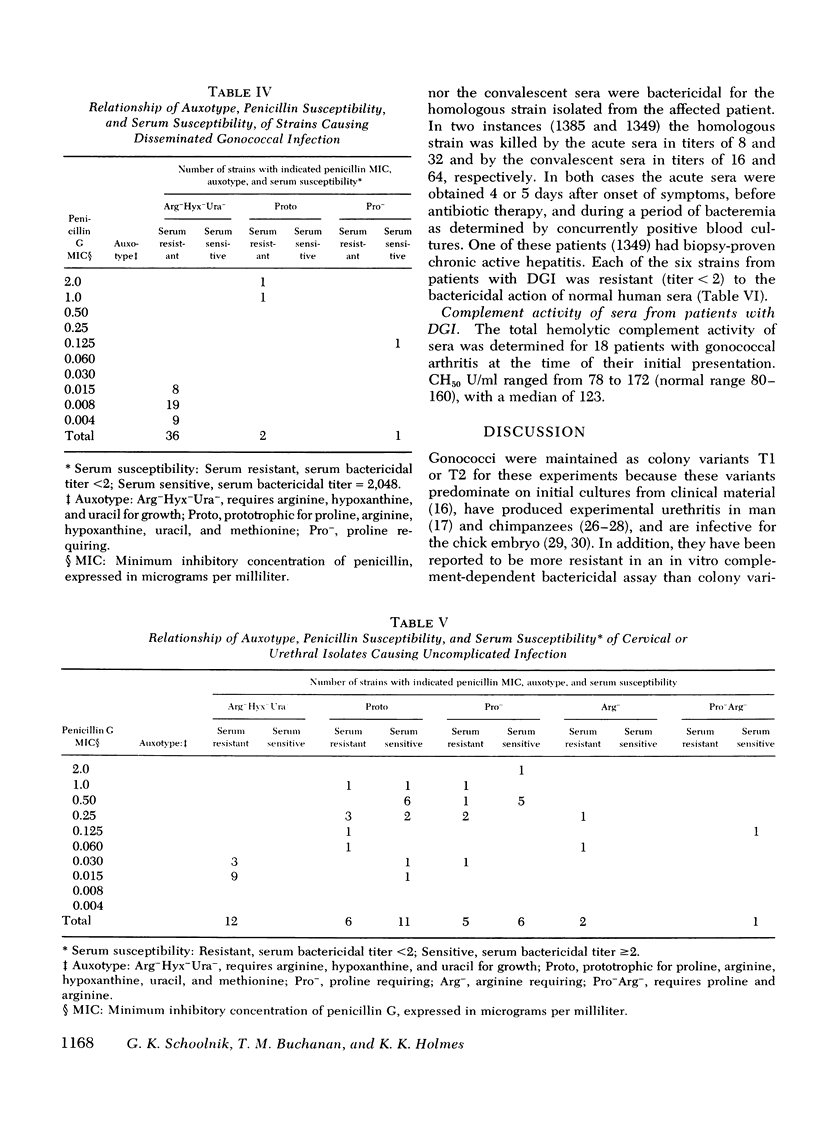
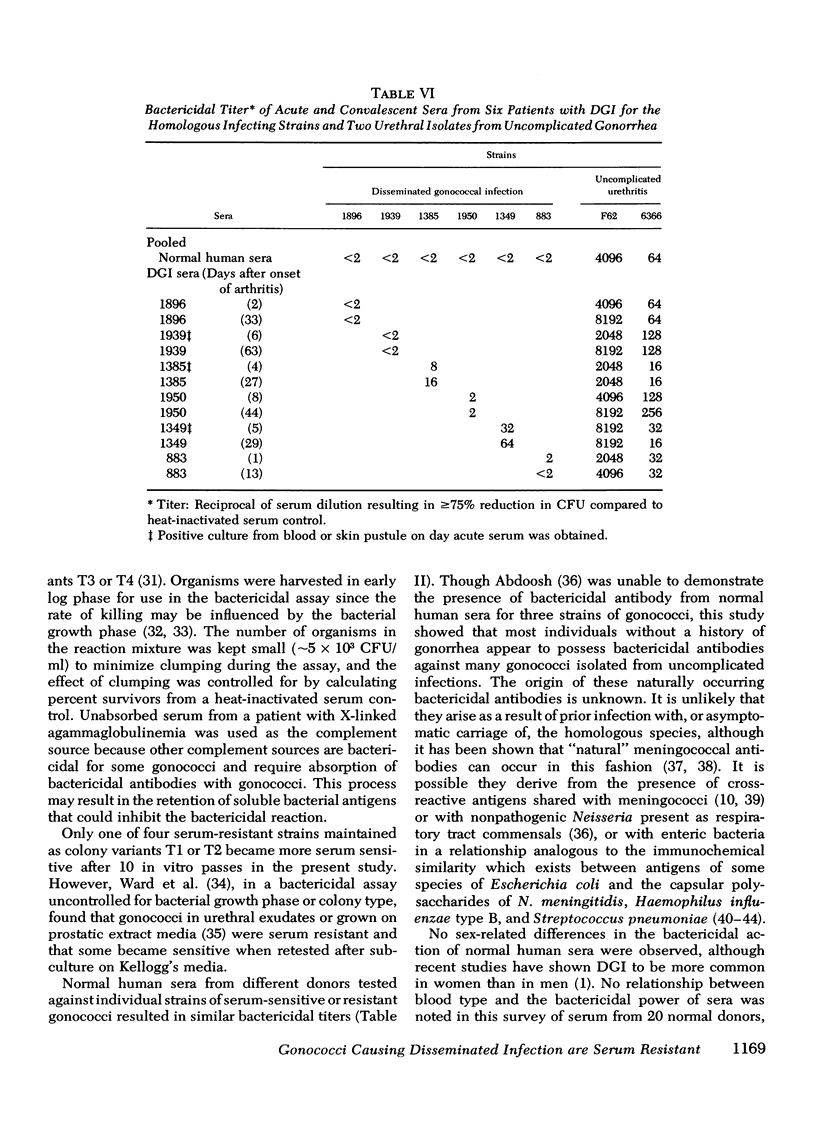
The susceptibility of strains of Neisseria gonorrhoeae to the bactericidal action of normal human sera was determined for isolates from patients with disseminated gonococcal infection and uncomplicated gonorrhea. Serum susceptibility was correlated with penicillin susceptibility and auxotype. 38 of 39 strains (97%) of N. gonorrhoeae from Seattle patients with disseminated gonococcal infection were resistant to the complement-dependent bactericidal action of normal human sera. 36 of these were inhibited by less than or equal to mug/ml of penicillin G and required arginine, hypoxanthine, and uracil for growth on chemically defined medium (Arg-Hyx-Ura- auxotype). 12 of 43 isolates from patients with uncomplicated gonorrhea were also of the Arg-Hyx-Ura-auxotype, inhibited by less than or equal to 0.030 mug/ml of penicillin G, and serum resistant. Of the 31 remaining strains of other auxotypes isolated from patients with uncomplicated gonorrhea, 18 (58.1%) were sensitive to normal human sera in titers ranging from 2 to 2,048. The bactericidal action of normal human sera may prevent the dissemination of serum-sensitive gonococci. However, since only a small proportion of individuals infected by serum-resistant strains develop disseminated gonococcal infection, serum resistance appears to be a necessary but not a sufficient virulence factor for dissemination. Host factors such as menstruation and pharyngeal gonococcal infection may favor the dissemination of serum-resistant strains. Since serum-resistant Arg-Hyx-Ura strains are far more frequently isolated from patients with disseminated gonococcal infection than serum-resistant strains of other auxotypes, Arg-Hyx-Ura-strains may possess other virulence factors in addition to serum resistance.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A., Abramson N., Johnston R. B., Jr, Jandl J. H., Rosen F. S. Increased susceptibility to infection associated with abnormalities of complement-mediated functions and of the third component of complement (C3). N Engl J Med. 1970 Feb 12;282(7):350–354. doi: 10.1056/nejm197002122820701. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Bloch K. J., Rosen F. S. Increased susceptibility to infection in a patient with type II essential hypercatabolism of C3. N Engl J Med. 1973 Mar 22;288(12):601–606. doi: 10.1056/NEJM197303222881204. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Colten H. R., Rosen F. S., Rabson A. R., Macnab G. M., Gear J. S. Homozygous deficiency of C3 in a patient with repeated infections. Lancet. 1972 Dec 2;2(7788):1179–1181. doi: 10.1016/s0140-6736(72)92598-6. [DOI] [PubMed] [Google Scholar]
- Arko R. J., Kraus S. J., Brown W. J., Buchanan T. M., Kuhn U. S. Neisseria gonorrhoeae: effects of systemic immunization on resistance of chimpanzees to urethral infection. J Infect Dis. 1974 Aug;130(2):160–164. doi: 10.1093/infdis/130.2.160. [DOI] [PubMed] [Google Scholar]
- BRUTON O. C. Agammaglobulinemia. Pediatrics. 1952 Jun;9(6):722–728. [PubMed] [Google Scholar]
- Brown W. J., Lucas C. T., Kuhn U. S. Gonorrhoea in the chimpanzee. Infection with laboratory-passed gonococci and by natural transmission. Br J Vener Dis. 1972 Jun;48(3):177–178. doi: 10.1136/sti.48.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Gotschlich E. C. Studies on gonococcus infection. 3. Correlation of gonococcal colony morphology with infectivity for the chick embryo. J Exp Med. 1973 Jan 1;137(1):196–200. doi: 10.1084/jem.137.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Swanson J., Holmes K. K., Kraus S. J., Gotschlich E. C. Quantitative determination of antibody to gonococcal pili. Changes in antibody levels with gonococcal infection. J Clin Invest. 1973 Nov;52(11):2896–2909. doi: 10.1172/JCI107486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumgarner L. R., Finkelstein R. A. Pathogenesis and immunology of experimental gonococcal infection: virulence of colony types of Neisseria gonorrhoeae for chicken embryos. Infect Immun. 1973 Dec;8(6):919–924. doi: 10.1128/iai.8.6.919-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carifo K., Catlin B. W. Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media. Appl Microbiol. 1973 Sep;26(3):223–230. doi: 10.1128/am.26.3.223-230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- DAVIS S. D., WEDGWOOD R. J. KINETICS OF THE BACTERICIDAL ACTION OF NORMAL SERUM ON GRAM-NEGATIVE BACTERIA. J Immunol. 1965 Jul;95:75–79. [PubMed] [Google Scholar]
- Fudenberg H., Good R. A., Goodman H. C., Hitzig W., Kunkel H. G., Roitt I. M., Rosen F. S., Rowe D. S., Seligmann M., Soothill J. R. Primary immunodeficiencies. Report of a World Health Organization Committee. Pediatrics. 1971 May;47(5):927–946. [PubMed] [Google Scholar]
- GOOD R. A., VARCO R. L. A clinical and experimental study of agammaglobulinemia. J Lancet. 1955 Jun;75(6):245–271. [PubMed] [Google Scholar]
- GREAVES R. I. Preservation of living cells by freeze-drying. Ann N Y Acad Sci. 1960 Apr 13;85:723–728. doi: 10.1111/j.1749-6632.1960.tb49992.x. [DOI] [PubMed] [Google Scholar]
- Glynn A. A., Ward M. E. Nature and Heterogeneity of the Antigens of Neisseria gonorrhoeae Involved in the Serum Bactericidal Reaction. Infect Immun. 1970 Aug;2(2):162–168. doi: 10.1128/iai.2.2.162-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grados O., Ewing W. H. Antigenic relationship between Escherichia coli and Neisseria meningitidis. J Infect Dis. 1970 Jul-Aug;122(1):100–103. doi: 10.1093/infdis/122.1-2.100. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- HESS E. V., HUNTER D. K., ZIFF M. GONOCOCCAL ANTIBODIES IN ACUTE ARTHRITIS. JAMA. 1965 Feb 15;191:531–534. doi: 10.1001/jama.1965.03080070015004. [DOI] [PubMed] [Google Scholar]
- Holmes K. K., Counts G. W., Beaty H. N. Disseminated gonococcal infection. Ann Intern Med. 1971 Jun;74(6):979–993. doi: 10.7326/0003-4819-74-6-979. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Winkelhake J. L., Zollinger W. D., Brandt B. L., Artenstein M. S. Immunochemical similarity between polysaccharide antigens of Escherichia coli 07: K1(L):NM and group B Neisseria meningitidis. J Immunol. 1973 Jan;110(1):262–268. [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- Leddy J. P., Frank M. M., Gaither T., Baum J., Klemperer M. R. Hereditary deficiency of the sixth component of complement in man. I. Immunochemical, biologic, and family studies. J Clin Invest. 1974 Feb;53(2):544–553. doi: 10.1172/JCI107588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C. T., Chandler F., Jr, Martin J. E., Jr, Schmale J. D. Transfer of gonococcal urethritis from man to chimpanzee. An animal model for gonorrhea. JAMA. 1971 Jun 7;216(10):1612–1614. [PubMed] [Google Scholar]
- MUSCHEL L. H. Serum bactericidal actions. Ann N Y Acad Sci. 1960 Nov 21;88:1265–1272. doi: 10.1111/j.1749-6632.1960.tb20117.x. [DOI] [PubMed] [Google Scholar]
- Miller M. E., Nilsson U. R. A familial deficiency of the phagocytosis-enhancing activity of serum related to a dysfunction of the fifth component of complement (C5). N Engl J Med. 1970 Feb 12;282(7):354–358. doi: 10.1056/NEJM197002122820702. [DOI] [PubMed] [Google Scholar]
- Myerowitz R. L., Handzel Z. T., Scheerson R., Robbins J. B. Induction of Haemophilus influenzae type b capsular antibody in neonatal rabbits by gastrointestinal colonization with cross-reacting Escherichia coli. Infect Immun. 1973 Feb;7(2):137–140. doi: 10.1128/iai.7.2.137-140.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B. H., Graham J. A., Brooks G. F. Human deficiency of the eighth component of complement. The requirement of C8 for serum Neisseria gonorrhoeae bactericidal activity. J Clin Invest. 1976 Feb;57(2):283–290. doi: 10.1172/JCI108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Bearn A. G. Genetic aspects of diseases of complement: an explosion. Am J Med. 1975 Jan;58(1):105–111. doi: 10.1016/0002-9343(75)90540-9. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. The virulence of strains of Bacterium coli for mice. Br J Exp Pathol. 1954 Dec;35(6):528–538. [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D., WARDLAW A. C. Lysis of gram-negative bacteria by serum. J Gen Microbiol. 1958 Apr;18(2):529–533. doi: 10.1099/00221287-18-2-529. [DOI] [PubMed] [Google Scholar]
- Roantree R. J., Rantz L. A. A STUDY OF THE RELATIONSHIP OF THE NORMAL BACTERICIDAL ACTIVITY OF HUMAN SERUM TO BACTERIAL INFECTION. J Clin Invest. 1960 Jan;39(1):72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., Myerowitz L., Whisnant J. K., Argaman M., Schneerson R., Handzel Z. T., Gotschlich E. C. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and 3. Infect Immun. 1972 Nov;6(5):651–656. doi: 10.1128/iai.6.5.651-656.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. G., Tolchin D., Halpern C. Enteric bacterial agents and the ABO blood groups. Am J Hum Genet. 1971 Mar;23(2):135–145. [PMC free article] [PubMed] [Google Scholar]
- Rowley D. Endotoxins and bacterial virulence. J Infect Dis. 1971 Mar;123(3):317–327. doi: 10.1093/infdis/123.3.317. [DOI] [PubMed] [Google Scholar]
- Rowley D. Endotoxins and bacterial virulence. J Infect Dis. 1971 Mar;123(3):317–327. doi: 10.1093/infdis/123.3.317. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Bradshaw M., Whisnant J. K., Myerowitz R. L., Parke J. C., Jr, Robbins J. B. An Escherichia coli antigen cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b: occurrence among known serotypes, and immunochemical and biologic properties of E. coli antisera toward H. influenzae type b. J Immunol. 1972 Jun;108(6):1551–1562. [PubMed] [Google Scholar]
- Spink W. W., Keefer C. S. STUDIES OF GONOCOCCAL INFECTION. I. A STUDY OF THE MODE OF DESTRUCTION OF THE GONOCOCCUS IN VITRO. J Clin Invest. 1937 Mar;16(2):169–176. doi: 10.1172/JCI100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink W. W., Keefer C. S. STUDIES OF GONOCOCCAL INFECTION. II. THE BACTERIOLYTIC POWER OF THE WHOLE DEFIBRINATED BLOOD OF PATIENTS WITH GONOCOCCAL ARTHRITIS. J Clin Invest. 1937 Mar;16(2):177–183. doi: 10.1172/JCI100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J. D., Martin J. E., Jr Improved medium selective for cultivation of N. gonorrhoeae and N. meningitidis. Public Health Rep. 1966 Jun;81(6):559–562. [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Artenstein M. S. Cross-reactivity of Neisseria gonorrhoeae and Neisseria meningitidis and the nature of antigens involved in the bactericidal reaction. J Infect Dis. 1974 Sep;130(3):240–247. doi: 10.1093/infdis/130.3.240. [DOI] [PubMed] [Google Scholar]
- Vosti K. L., Randall E. Sensitivity of serologically classified strains of escherichia coli of human origin to the serum bactericidal system. Am J Med Sci. 1970 Feb;259(2):114–119. doi: 10.1097/00000441-197002000-00005. [DOI] [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Glynn A. A. Gonococci in urethral exudates possess a virulence factor lost on subculture. Nature. 1970 Jul 25;227(5256):382–384. doi: 10.1038/227382a0. [DOI] [PubMed] [Google Scholar]
- Watt P. J., Glynn A. A., Ward M. E. Maintenance of virulent gonococci in laboratory culture. Nat New Biol. 1972 Apr 12;236(67):186–187. doi: 10.1038/newbio236186a0. [DOI] [PubMed] [Google Scholar]
- Wiesner P. J., Handsfield H. H., Holmes K. K. Low antibiotic resistance of gonococci causing disseminated infection. N Engl J Med. 1973 Jun 7;288(23):1221–1222. doi: 10.1056/NEJM197306072882308. [DOI] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]



