Abstract
It has been suggested that the hyperglucagonemia observed in diabetic animals and man may be due to an impairment of glucose uptake and metabolism by the alpha-cells resulting in a decreased production of ATP. To test this hypothesis glucose, ATP, glucagon, and insulin were measured in pancreatic islets of normal and alloxan or streptozotocin diabetic rats. Two experimental approaches were used. In the first, the pancreas was perfused in vitro for assessing insulin and glucagon release due to 10 mM amino acids with and without 5 mM glucose. These perfusions were performed in the presence and absence of insulin. After perfusion, the pancreas was frozen and processed for analysis of islet glucose, ATP, insulin, and glucagon content. The second approach was to investigate the islet sucrose, urea, and glucose spaces together with ATP, insulin, and glucagon content in vivo in normal and in insulin-treated and untreated streptozotocin diabetic rats. Perfusion of the pancreas in vitro with 5 mM glucose resulted in higher glucose content of normal islets than in alloxan and streptozotocin diabetic islets. Similarly in the in vivo studies, the intracellular glucose space of the streptozotocin diabetic islets was 30% the value found in normals. In the in vivo experiments, despite the relatively small intracellular glucose space of alpha-cell islets, the ATP content of these islets was only 15-20% lower than the ATP content of normal islets. In the in vitro experiments, perfusion with glucose resulted in ATP contents of alpha-cell islets and of normal mixed alpha-beta-cell islets which were indistinguishable. However, the ATP content of alpha-cell islets was maintained for prolonged periods in the absence of glucose in contrast to mixed islets, composed primarily of beta-cells, in which the ATP level decreased by 45% when glucose-free medium was perfused for sustained periods. Finally, insulin infused in high concentrations or administered to the diabetic animal had no effect on the glucose spaces or the ATP contents of normal or alpha-cell islets. It can be calculated that in vivo the intracellular glucose level of islets from streptozotocin treated rats is approximately 15 mM. Since in normals an extracellular glucose concentration of this magnitude inhibits stimulated glucagon release completely, it would seem unlikely that a lack of intracellular glucose is the cause of the apparent glucose "blindness" of the alpha-cells in diabetes. In fact, in perfusion studies as little as 2.5 mM free intracellular glucose was sufficient to suppress glucagon secretion from diabetic alpha-cells. The results of the ATP measurements clearly eliminate a possible energy deficit of diabetic alpha-cells as cause of the apparent glucose resistance of alpha-cells.
Full text
PDF
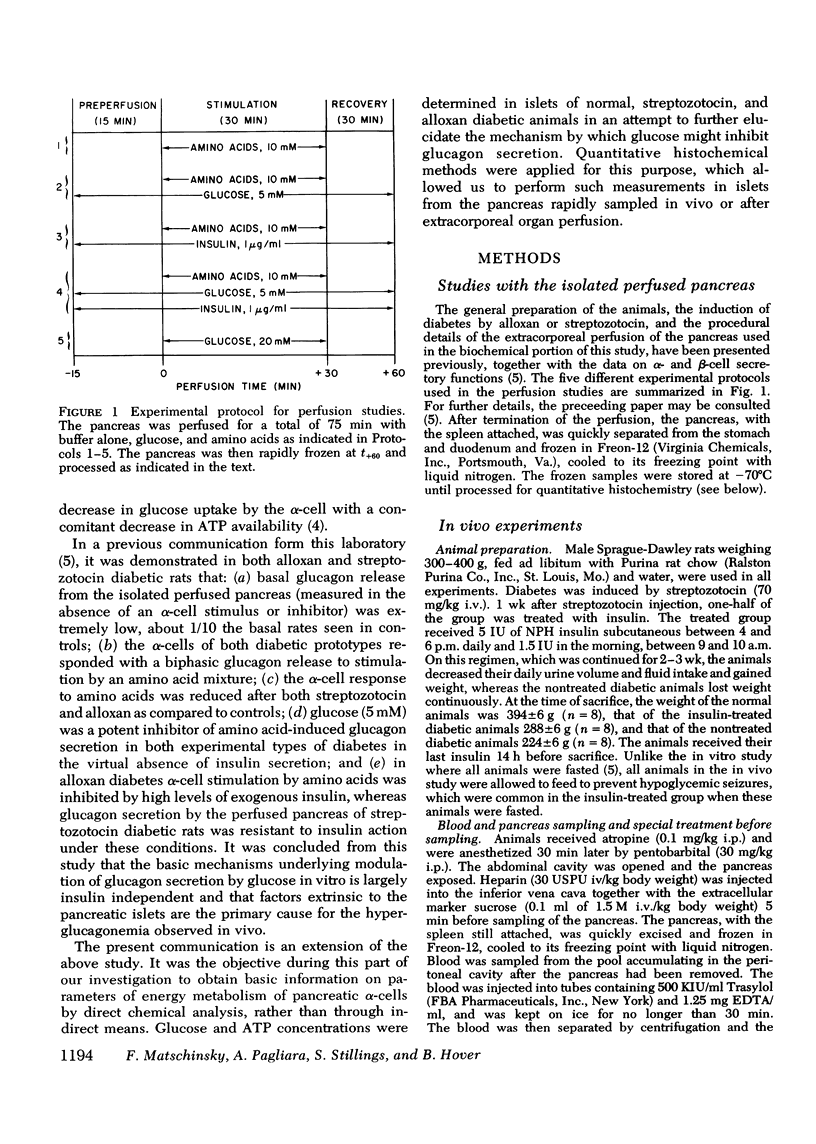
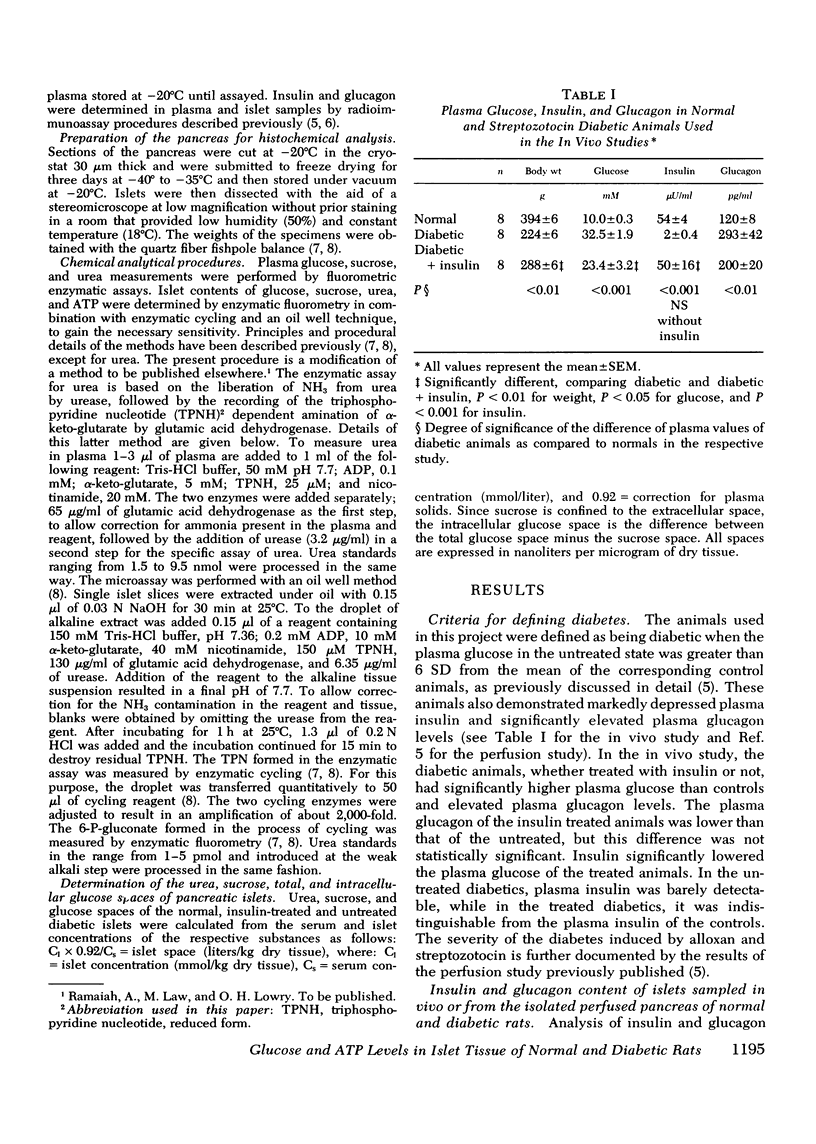
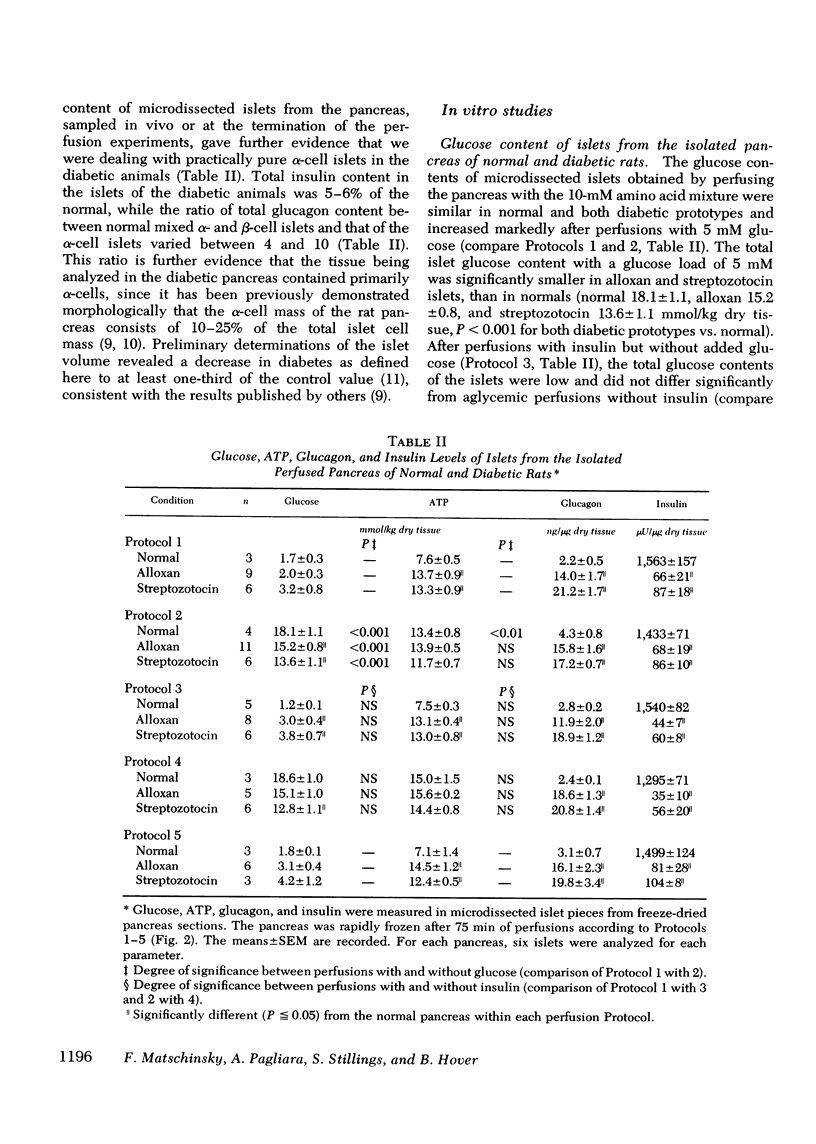
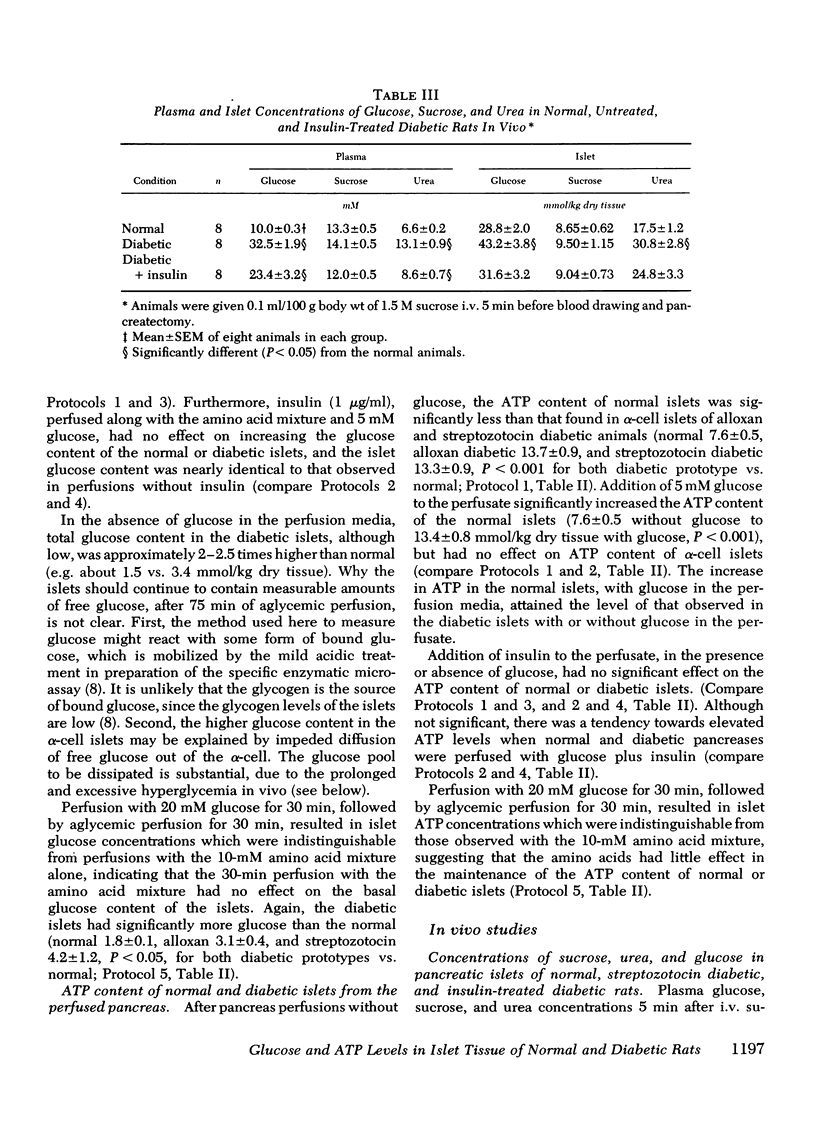


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edwards J. C., Taylor K. W. Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro. Biochim Biophys Acta. 1970 Aug 14;215(2):310–315. doi: 10.1016/0304-4165(70)90029-2. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Charles M. A., Grodsky G. M. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest. 1974 Oct;54(4):833–841. doi: 10.1172/JCI107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D. A., Matschinsky F. M. Enzymes of the cholinergic system in islets of Langerhans. J Histochem Cytochem. 1975 Sep;23(9):645–651. doi: 10.1177/23.9.126256. [DOI] [PubMed] [Google Scholar]
- Hoftiezer V., Carpenter A. M. Comparison of streptozotocin and alloxan-induced diabetes in the rat, including volumetric quantitation of the pancreatic islets. Diabetologia. 1973 Jun;9(3):178–184. doi: 10.1007/BF01219780. [DOI] [PubMed] [Google Scholar]
- Leichter S. B., Pagliara A. S., Grieder M. H., Pohl S., Rosai J., Kipnis D. M. Uncontrolled diabetes mellitus and hyperglucagonemia associated with an islet cell carcinoma. Am J Med. 1975 Feb;58(2):285–293. doi: 10.1016/0002-9343(75)90579-3. [DOI] [PubMed] [Google Scholar]
- Matschinsky F. M., Passonneau J. V., Lowry O. H. Quantitative histochemical analysis of glycolytic intermediates and cofactors with an oil well technique. J Histochem Cytochem. 1968 Jan;16(1):29–39. doi: 10.1177/16.1.29. [DOI] [PubMed] [Google Scholar]
- Pagliara A. S., Stillings S. N., Haymond M. W., Hover B. A., Matschinsky F. M. Insulin and glucose as modulators of the amino acid-induced glucagon release in the isolated pancreas of alloxan and streptozotocin diabetic rats. J Clin Invest. 1975 Feb;55(2):244–255. doi: 10.1172/JCI107928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara A. S., Stillings S. N., Hover B., Martin D. M., Matschinsky F. M. Glucose modulation of amino acid-induced glucagon and insulin release in the isolated perfused rat pancreas. J Clin Invest. 1974 Oct;54(4):819–832. doi: 10.1172/JCI107822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin P., Fujita Y., Unger R. H. Effect of insulin-glucose infusions on plasma glucagon levels in fasting diabetics and nondiabetics. J Clin Invest. 1975 Nov;56(5):1132–1138. doi: 10.1172/JCI108188. [DOI] [PMC free article] [PubMed] [Google Scholar]



