Abstract
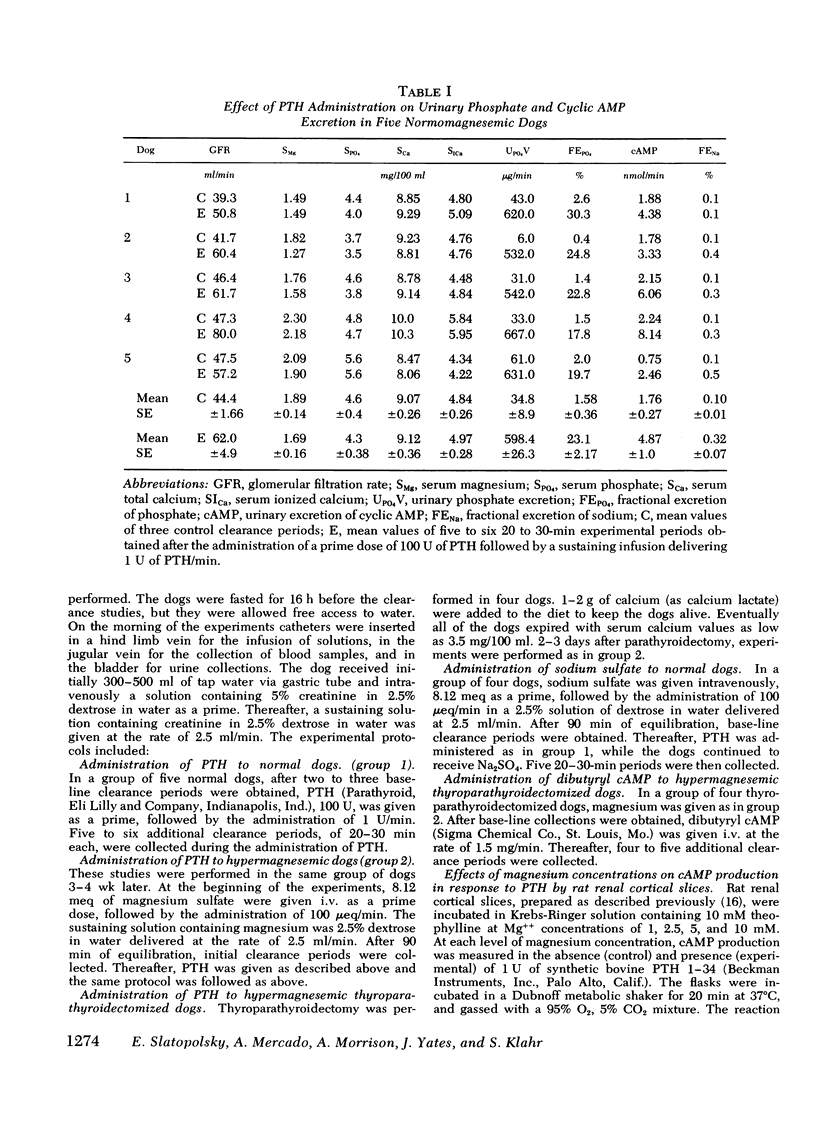
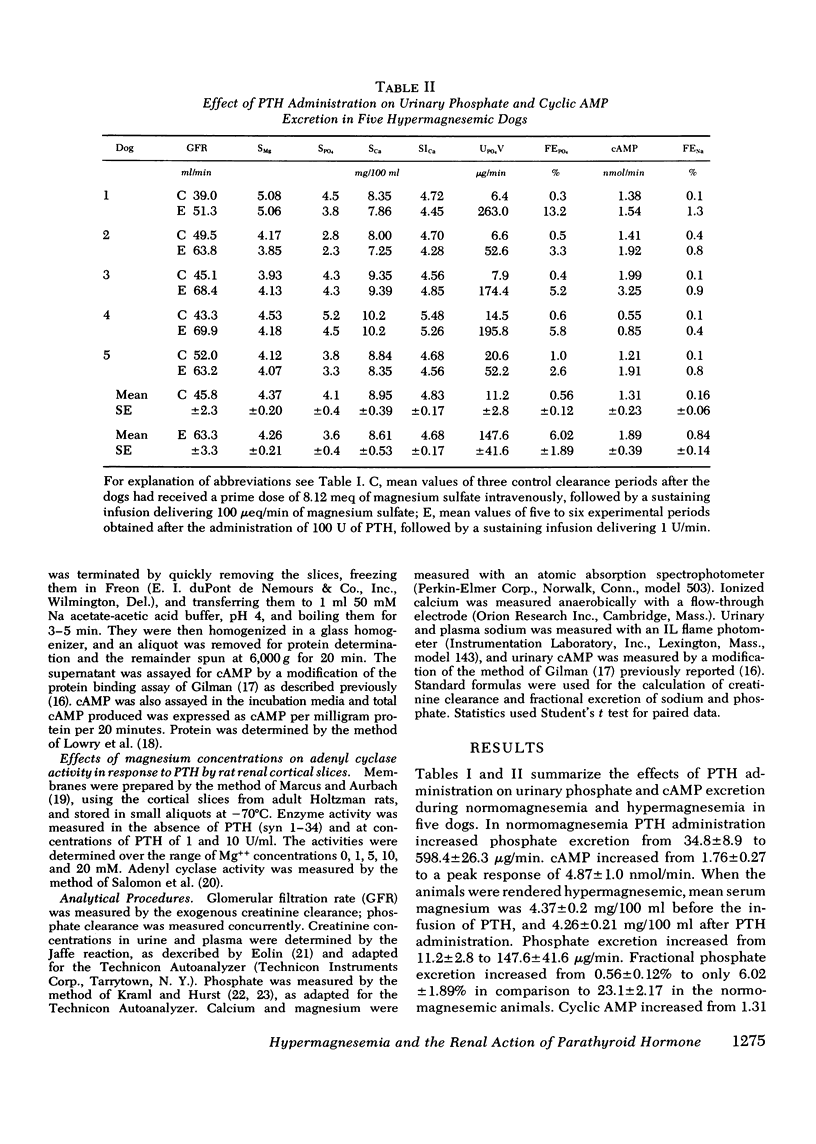
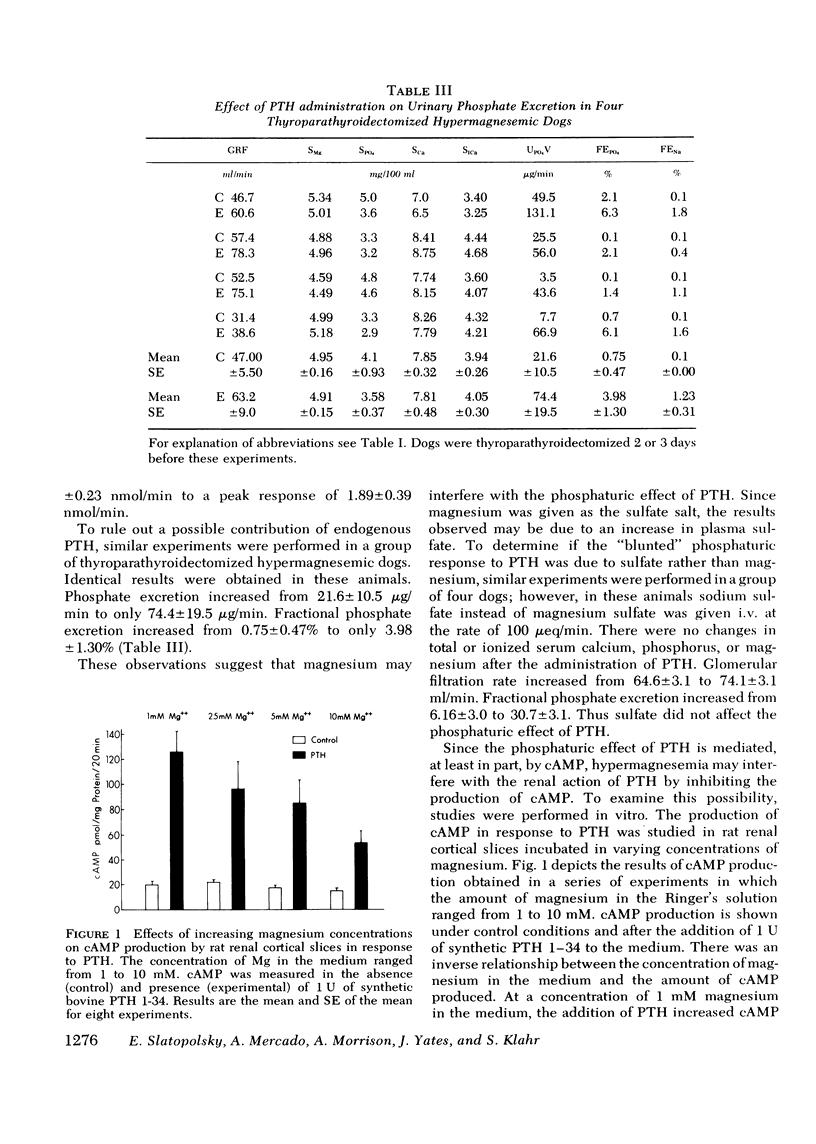
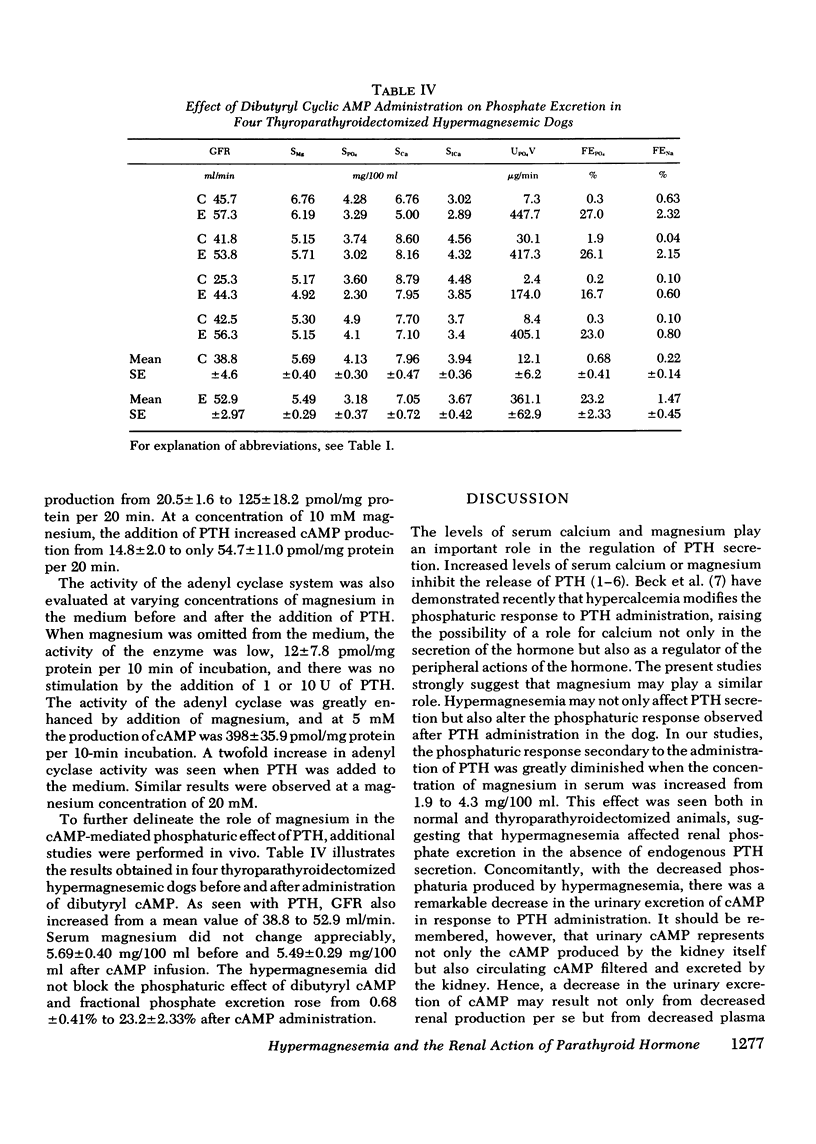
Available evidence indicates that serum magnesium (Mg++) levels influence the secretion rate of parathyroid hormone (PTH). Whether serum Mg++ concentrations also modify the action of PTH on its target organs has not been definitively established. The present experiments were designed to study this possibility. The effect of infusing PTH on the urinary excretion of cyclic AMP (cAMP) and PO4= was examined in five normal dogs at two different levels of serum Mg++. At normal serum Mg++ concentrations (1.89 +/- 0.14 mg/100 ml), PTH infusion increased cAMP excretion from 1.76 +/- 0.27 to 4.87 +/- 1.00 nmol/min and fractional PO4= excretion (FEPO4) from 1.58 +/- 0.36% to 23.1 +/- 2.17%. When an identical amount of PTH was given to the same dogs at a serum Mg++ of 4.36 +/- 0.20 mg/100 ml, FEPO4 increased to only 6.02+/-1.89% and cAMP from 1.31 +/- 0.23 to 1.89 +/- 0.39 nmol/min. Identical results were obtained in thyroparathyroidectomized hypermagnesemic dogs. Increased serum Mg++ levels had no effect on the phosphaturia produced by the infusion of dibutyryl cAMP to thyroparathyroidectomized dogs. In vitro studies using rat renal cortical slices revealed a progressive decrease in cAMP production in response to PTH as the Mg++ concentrations were increased in the incubation medium. The overall results indicate that hypermagnesemia inhibits the phosphaturic response to PTH by decreasing the renal production of cAMP. Plasma magnesium, therefore, may participate in a double feedback mechanism, not only controlling the release of PTH, but also altering the biological activity of the hormone at the level of the target organ.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anast C. S., Mohs J. M., Kaplan S. L., Burns T. W. Evidence for parathyroid failure in magnesium deficiency. Science. 1972 Aug 18;177(4049):606–608. doi: 10.1126/science.177.4049.606. [DOI] [PubMed] [Google Scholar]
- Arnaud C. D., Jr, Tenenhouse A. M., Rasmussen H. Parathyroid hormone. Annu Rev Physiol. 1967;29:349–372. doi: 10.1146/annurev.ph.29.030167.002025. [DOI] [PubMed] [Google Scholar]
- Aurbach G. D., Chase L. R. Cyclic 3',5'-adenylic acid in bone and the mechanism of action of parathyroid hormone. Fed Proc. 1970 May-Jun;29(3):1179–1182. [PubMed] [Google Scholar]
- Beck N., Singh H., Reed S. W., Davis B. B. Direct inhibitory effect of hypercalcemia on renal actions of parathyroid hormone. J Clin Invest. 1974 Mar;53(3):717–725. doi: 10.1172/JCI107610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle R. M., Care A. D., Cooper C. W., Gitelman H. J. The influence of plasma magnesium concentration on parathyroid hormone secretion. J Endocrinol. 1968 Dec;42(4):529–534. doi: 10.1677/joe.0.0420529. [DOI] [PubMed] [Google Scholar]
- Care A. D., Sherwood L. M., Potts J. T., Jr, Aurbach G. D. Perfusion of the isolated parathyroid gland of the goat and sheep. Nature. 1966 Jan 1;209(5018):55–57. doi: 10.1038/209055a0. [DOI] [PubMed] [Google Scholar]
- Chase L. R., Slatopolsky E. Secretion and metabolic efficacy of parthyroid hormone in patients with severe hypomagnesemia. J Clin Endocrinol Metab. 1974 Mar;38(3):363–371. doi: 10.1210/jcem-38-3-363. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Properties of cyclic 3',5'-nucleotide phosphodiesterase from rat brain. Biochemistry. 1967 Apr;6(4):1079–1087. doi: 10.1021/bi00856a017. [DOI] [PubMed] [Google Scholar]
- Connor T. B., Toskes P., Mahaffey J., Martin L. G., Williams J. B., Walser M. Parathyroid function during chronic magnesium deficiency. Johns Hopkins Med J. 1972 Aug;131(2):100–117. [PubMed] [Google Scholar]
- DRUMMOND G. I., PERROTT-YEE S. Enzymatic hydrolysis of adenosine 3',5'-phosphoric acid. J Biol Chem. 1961 Apr;236:1126–1129. [PubMed] [Google Scholar]
- Estep H., Shaw W. A., Watlington C., Hobe R., Holland W., Tucker S. G. Hypocalcemia due to hypomagnesemia and reversible parathyroid hormone unresponsiveness. J Clin Endocrinol Metab. 1969 Jun;29(6):842–848. doi: 10.1210/jcem-29-6-842. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Potts J. T., Jr Relative effectiveness of magnesium and calcium on the secretion and biosynthesis of parathyroid hormone in vitro. Endocrinology. 1976 Jan;98(1):197–202. doi: 10.1210/endo-98-1-197. [DOI] [PubMed] [Google Scholar]
- Hahn T. J., Chase L. R., Avioli L. V. Effect of magnesium depletion on responsiveness to parathyroid hormone in parathyroidectomized rats. J Clin Invest. 1972 Apr;51(4):886–891. doi: 10.1172/JCI106883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst R. O. A simplified approach to the use of determinants in the calculation of the rat equation for a complex enzyme system. Can J Biochem. 1967 Dec;45(12):2015–2039. doi: 10.1139/o67-235. [DOI] [PubMed] [Google Scholar]
- Kraml M. A semi-automated determination of phospholipids. Clin Chim Acta. 1966 Apr;13(4):442–448. doi: 10.1016/0009-8981(66)90235-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marcus R., Aurbach G. D. Bioassay of parathyroid hormone in vitro with a stable preparation of adenyl cyclase from rat kidney. Endocrinology. 1969 Nov;85(5):801–810. doi: 10.1210/endo-85-5-801. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Kleeman C. R. Evidence for suppression of parathyroid gland activity by hypermagnesemia. J Clin Invest. 1970 Sep;49(9):1619–1629. doi: 10.1172/JCI106379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldowney F. P., McKenna T. J., Kyle L. H., Freaney R., Swan M. Parathormone-like effect of magnesium replenishment in steatorrhea. N Engl J Med. 1970 Jan 8;282(2):61–68. doi: 10.1056/NEJM197001082820203. [DOI] [PubMed] [Google Scholar]
- Paunier L., Radde I. C., Kooh S. W., Conen P. E., Fraser D. Primary hypomagnesemia with secondary hypocalcemia in an infant. Pediatrics. 1968 Feb;41(2):385–402. [PubMed] [Google Scholar]
- Peck W. A., Carpenter J., Messinger K., DeBra D. Cyclic 3'5'-adenosine monophosphate in isolated bone cells. Response to low concentrations of parathyroid hormone. Endocrinology. 1973 Mar;92(3):692–697. doi: 10.1210/endo-92-3-692. [DOI] [PubMed] [Google Scholar]
- Rodriguez H. J., Walls J., Yates J., Klahr S. Effects of acetazolamide on the urinary excretion of cyclic AMP and on the activity of renal adenyl cyclase. J Clin Invest. 1974 Jan;53(1):122–130. doi: 10.1172/JCI107529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sherwood L. M., Potts J. T., Jr, Care A. D., Mayer G. P., Aurbach G. D. Evaluation by radioimmunoassay of factors controlling the secretion of parathyroid hormone. Nature. 1966 Jan 1;209(5018):52–55. doi: 10.1038/209052a0. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Johnston C. C., Jr Hormonal responsiveness of adenylate cyclase activity from separate bone cells. Endocrinology. 1974 Jul;95(1):130–139. doi: 10.1210/endo-95-1-130. [DOI] [PubMed] [Google Scholar]
- Suh S. M., Tashjian A. H., Jr, Matsuo N., Parkinson D. K., Fraser D. Pathogenesis of hypocalcemia in primary hypomagnesemia: normal end-organ responsiveness to parathyroid hormone, impaired parathyroid gland function. J Clin Invest. 1973 Jan;52(1):153–160. doi: 10.1172/JCI107159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard J. C., Webster P. D., Carr A. A. Primary hypomagnesemia with secondary hypocalcemia, diarrhea and insensitivity to parathyroid hormone. Am J Dig Dis. 1972 Jul;17(7):612–618. doi: 10.1007/BF02231747. [DOI] [PubMed] [Google Scholar]


