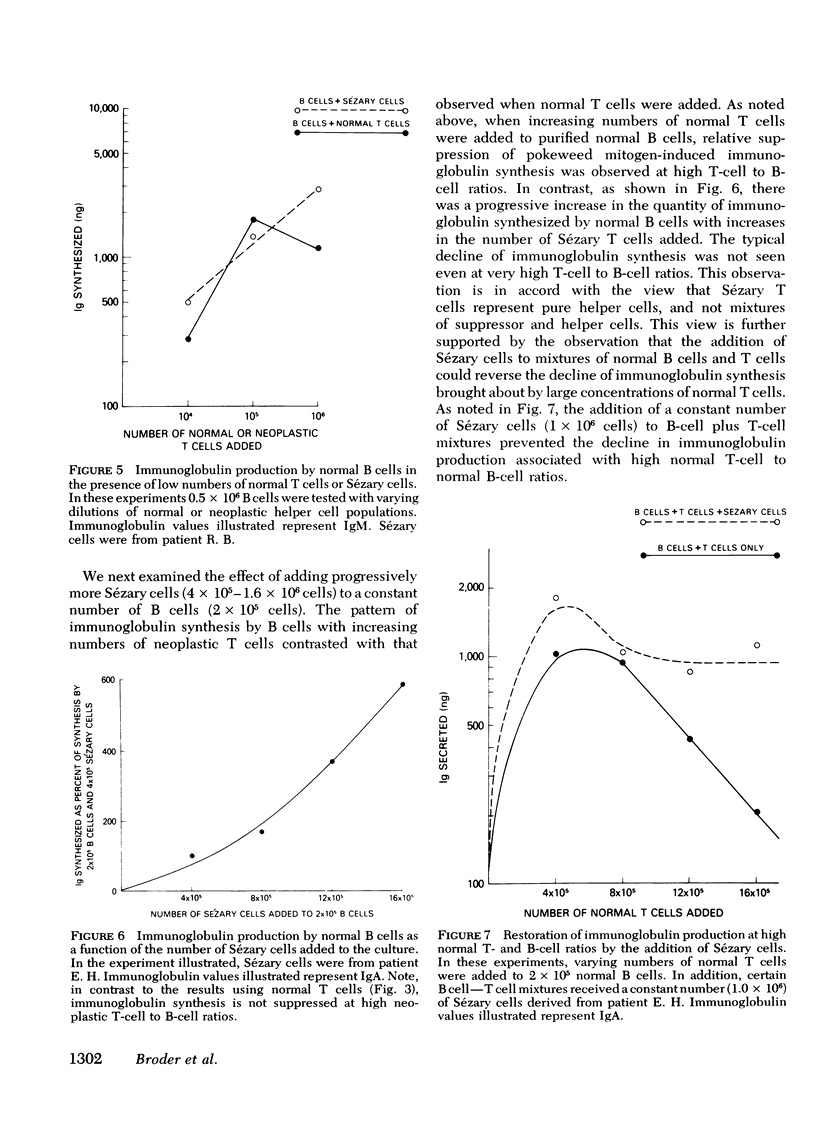
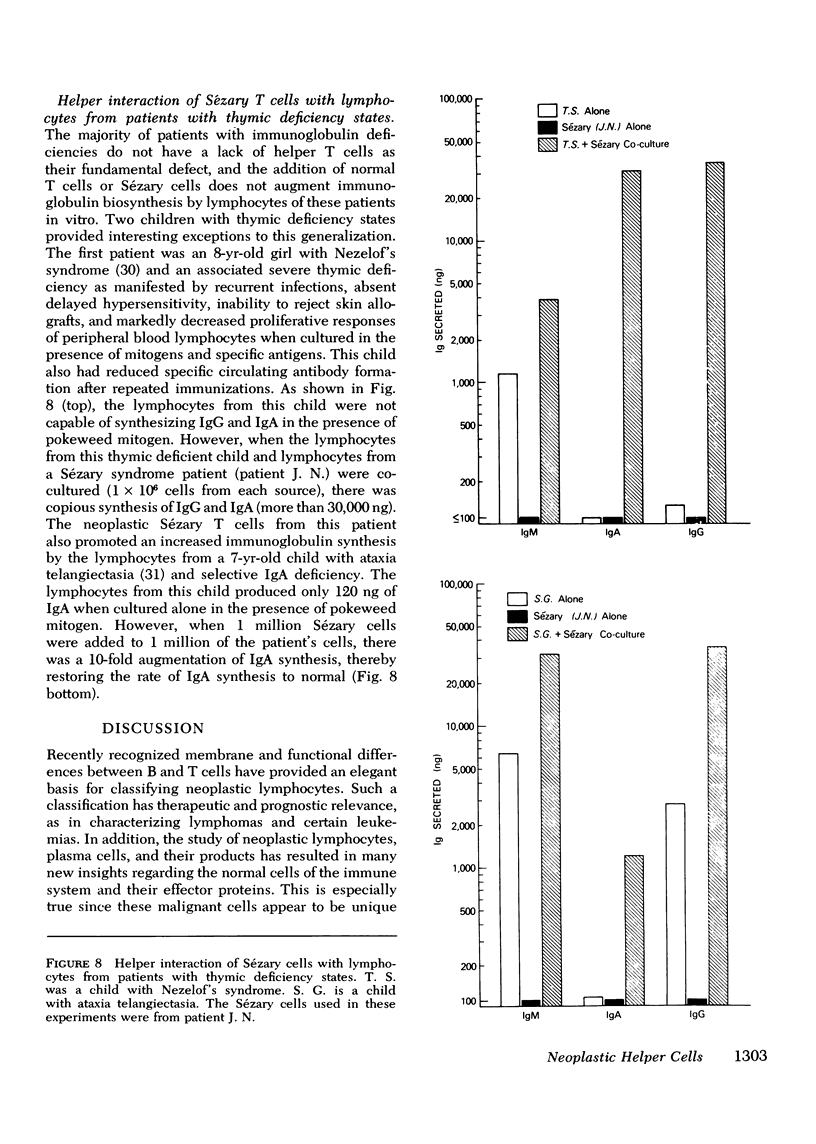
Abstract
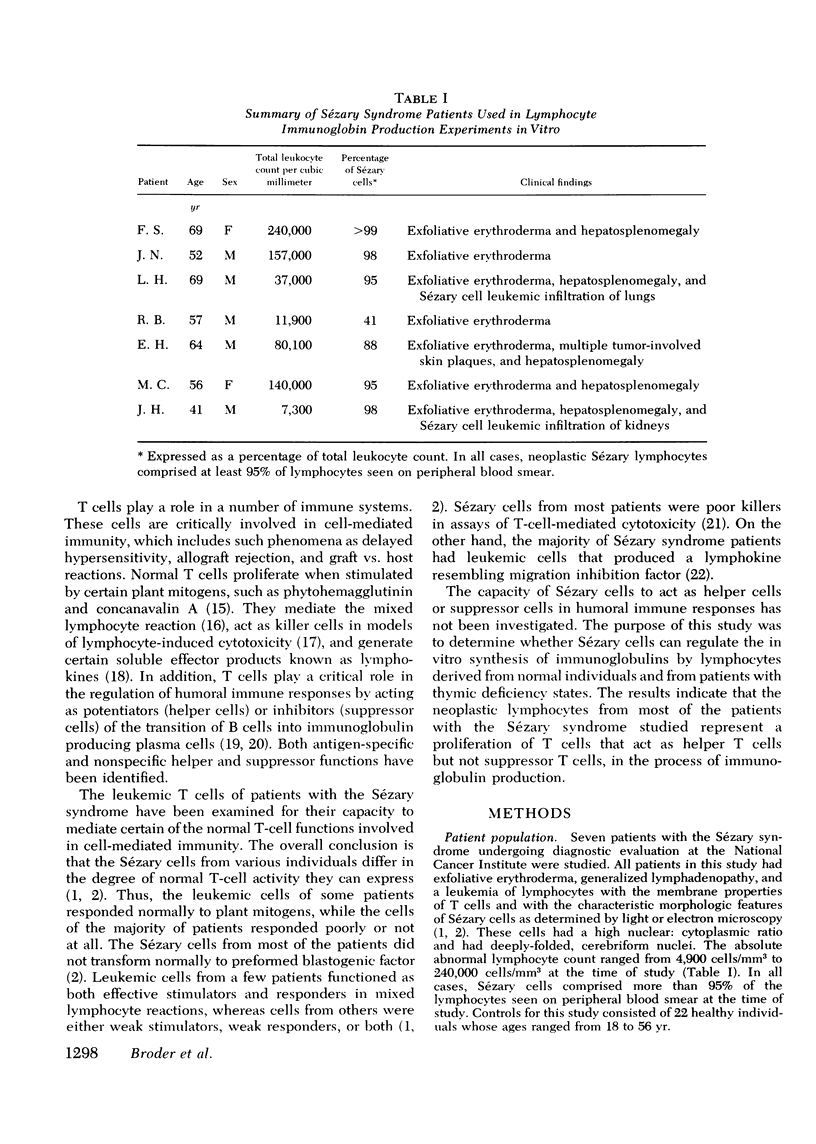
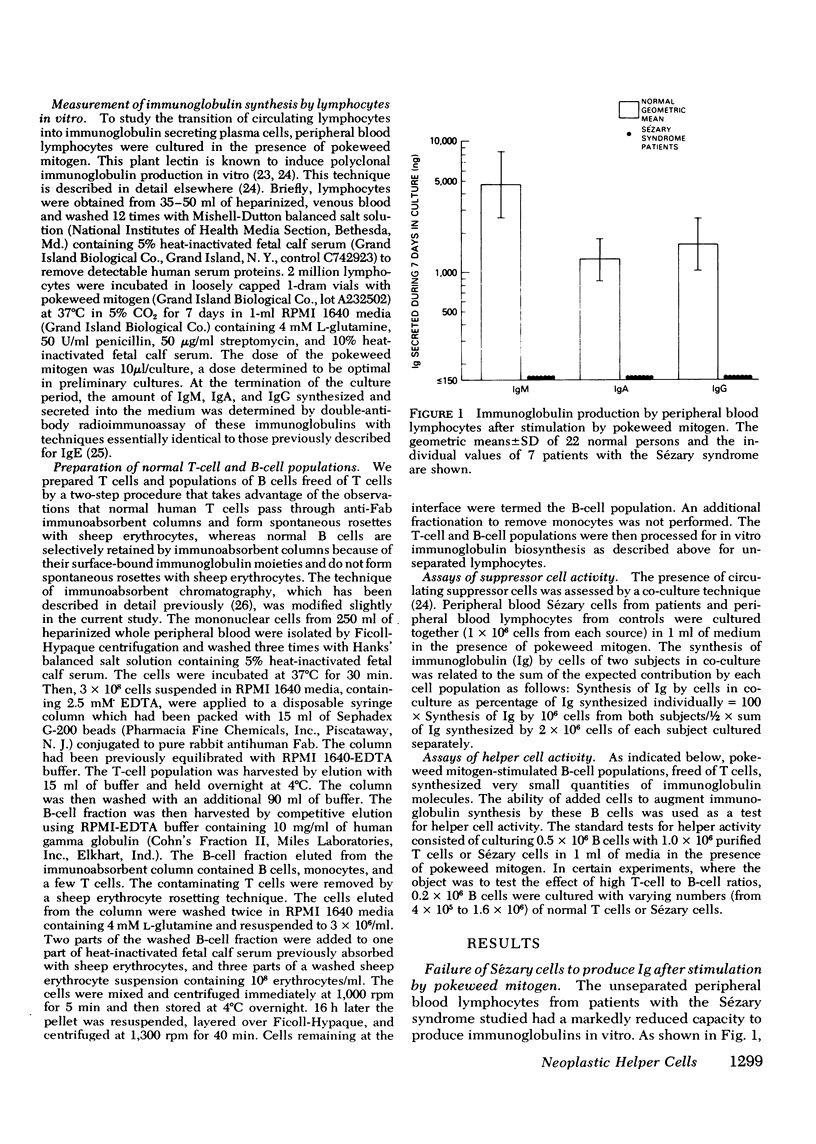
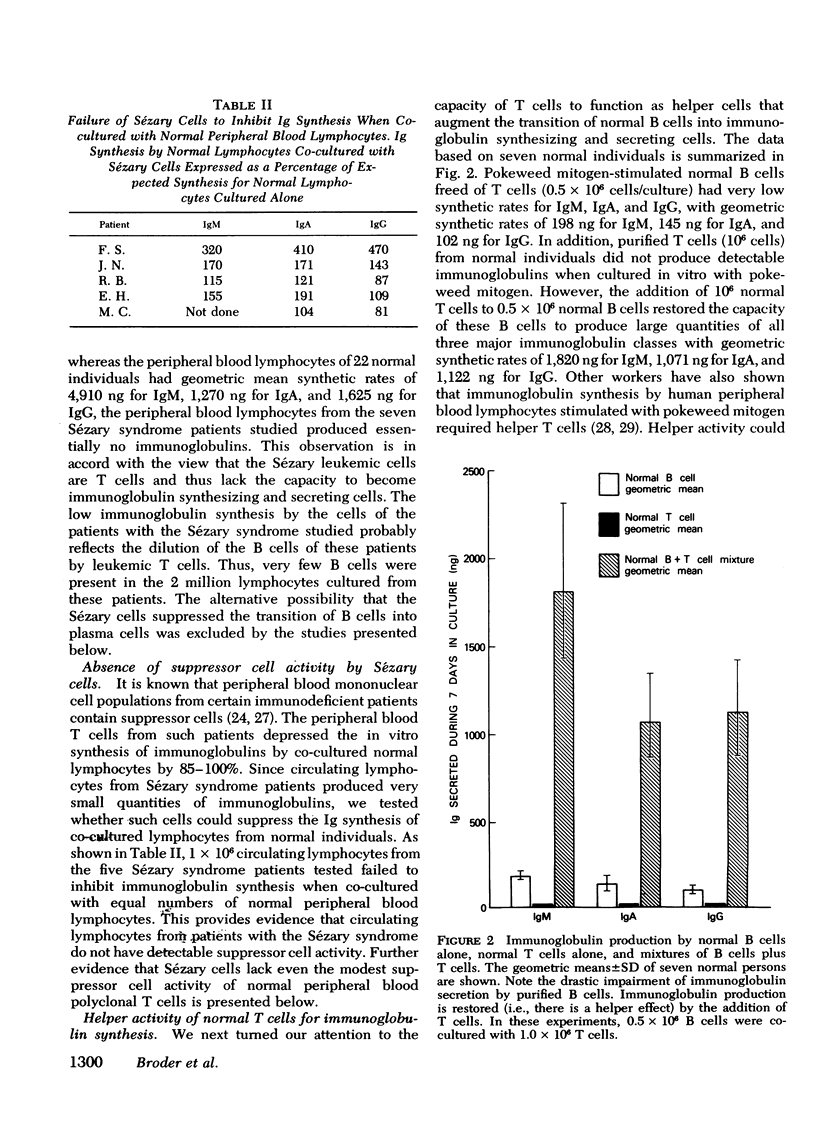
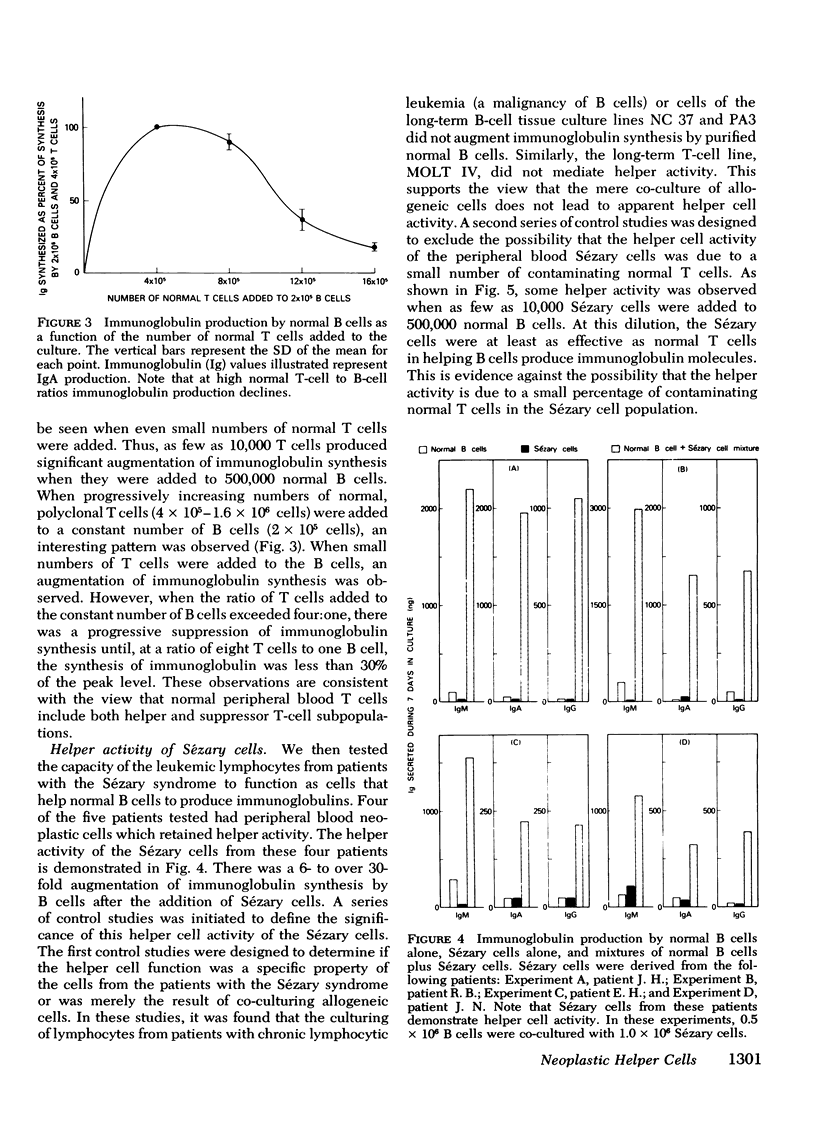
The Sézary syndrome is a frequently lethal disease characterized by circulating malignant cells of thymus-derived (T)-cell origin. The capacity of circulating malignant lymphocytes from patients with this syndrome to synthesize immunoglobulins and to function as helper or suppressor cells regulating immunoglobulin synthesis by bone marrow-derived (B) lymphocytes was determined. Peripheral blood lymphocytes from normal individuals had geometric mean immunoglobulin synthetic rates of 4,910 ng for IgM, 1,270 ng for IgA, and 1,625 ng for IgG per 2 X 10(6) cells in culture with pokeweed mitogen for 7 days. Purified normal B cells had geometric mean synthetic rates of 198 ng for IgM, 145 ng for IgA, and 102 ng for IgG. Leukemic cells from patients with the Sézary syndrome produced essentially no immunoglobulins. Adding normal T cells to normal B cells restored their immunoglobin producing capacity. Leukemic cells from four of five patients tested had a similar capacity to help immunoglobulin synthesis by purified normal B cells. Additionally, Sézary cells from one patient studied induced a nearly 10-fold increase in IgA synthesis by lymphocytes from a child with ataxia telangiectasia and selective IgA deficiency. Furthermore, these Sézary cells induced more than a 500-fold increase in IgG and IgA synthesis by lymphocytes from a child with Nezelof's syndrome. When Sézary cells were added to normal unfractionated lymphocytes, they did not suppress immunoglobulin biosynthesis. In addition, unlike the situation observed when large numbers of normal T cells were added to purified B cells, there was no depression of immunoglobulin synthesis at very high malignant T-cell to B-cell ratios. These data support the view that Sézary T cells do not express suppressor cell activity. The results presented in this paper suggest that neoplastic lymphocytes from the majority of patients with the Sézary syndrome originate from a subset of T cells programmed exclusively for helper-like interactions with B cells in their production of immunoglobulin molecules.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Bloch K. J. Immunoglobulins on the surface of neoplastic lymphocytes. N Engl J Med. 1972 Aug 10;287(6):272–276. doi: 10.1056/NEJM197208102870603. [DOI] [PubMed] [Google Scholar]
- Braylan R. C., Jaffe E. S., Berard C. W. Malignant lymphomas: current classification and new observations. Pathol Annu. 1975;10:213–270. [PubMed] [Google Scholar]
- Broom B. C., De la Concha E. G., Webster A. D., Janossy G. J., Asherson G. L. Intracellular immunoglobulin production in vitro by lymphocytes from patients with hypogammaglobulinaemia and their effect on normal lymphocytes. Clin Exp Immunol. 1976 Jan;23(1):73–77. [PMC free article] [PubMed] [Google Scholar]
- Brouet J. C., Flandrin G., Seligmann M. Indications of the thymus-derived nature of the proliferating cells in six patients with Sézary's syndrome. N Engl J Med. 1973 Aug 16;289(7):341–344. doi: 10.1056/NEJM197308162890703. [DOI] [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Coutinho A., Möller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- Dennert G. Thymus derived killer cells: specificity of function, and antigen recognition. Transplant Rev. 1976;29:59–88. doi: 10.1111/j.1600-065x.1976.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Dickler H. B., Kunkel H. G. Interaction of aggregated -globulin with B lymphocytes. J Exp Med. 1972 Jul 1;136(1):191–196. doi: 10.1084/jem.136.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson R. L., Kirkpatrick C. H., Shevach E. M., Schein P. S., Smith R. W., Green I., Lutzner M. Preferential cutaneous infiltration by neoplastic thymus-derived lymphocytes. Morphologic and functional studies. Ann Intern Med. 1974 Jun;80(6):685–692. doi: 10.7326/0003-4819-80-6-685. [DOI] [PubMed] [Google Scholar]
- Fröland S. S. Binding of sheep erythrocytes to human lymphocytes. A probable marker of T lymphocytes. Scand J Immunol. 1972;1(3):269–280. doi: 10.1111/j.1365-3083.1972.tb01818.x. [DOI] [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandinski J., Cantor H., Tadakuma T., Peavy D. L., Pierce C. W. Separation of helper T cells from suppressor T cells expressing different Ly components. I. Polyclonal activation: suppressor and helper activities are inherent properties of distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1382–1390. doi: 10.1084/jem.143.6.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. Functional analysis of murine and human B lymphocyte subsets. Transplant Rev. 1975;24:177–236. doi: 10.1111/j.1600-065x.1975.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrmann H. P., Whang-Peng J. Human mixed leukocyte culture: identification of the proliferating lymphocyte subpopulation by sex chromosome markers. J Exp Med. 1974 Jul 1;140(1):54–60. doi: 10.1084/jem.140.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzner M., Edelson R., Schein P., Green I., Kirkpatrick C., Ahmed A. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975 Oct;83(4):534–552. doi: 10.7326/0003-4819-83-4-534. [DOI] [PubMed] [Google Scholar]
- McFarlin D. E., Strober W., Waldmann T. A. Ataxia-telangiectasia. Medicine (Baltimore) 1972 Jul;51(4):281–314. doi: 10.1097/00005792-197207000-00002. [DOI] [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Surface bound immunoglobulins as a cell marker in human lymphoproliferative diseases. Blood. 1972 Dec;40(6):777–794. [PubMed] [Google Scholar]
- Smith R. W., Terry W. D., Buell D. N., Sell K. W. An antigenic marker for human thymic lymphocytes. J Immunol. 1973 Mar;110(3):884–887. [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Polmar S. H., Balestra S. T., Jost M. C., Bruce R. M., Terry W. D. Immunoglobulin E in immunologic deficiency diseases. II. Serum IgE concentration of patients with acquired hypogammaglobulinemia, thymoma and hypogammaglobulinemia, myotonic dystrophy, intestinal lymphangiectasia and Wiskott-Aldrich syndrome. J Immunol. 1972 Aug;109(2):304–310. [PubMed] [Google Scholar]
- Wu L. Y., Lawton A. R., Cooper M. D. Differentiation capacity of cultured B lymphocytes from immunodeficient patients. J Clin Invest. 1973 Dec;52(12):3180–3189. doi: 10.1172/JCI107518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Edelson R., Cohen S., Green I. Migration inhibitory activity in serum and cell supernatants in patients with Sezary syndrome. J Immunol. 1975 Mar;114(3):915–918. [PubMed] [Google Scholar]
- Yoshida T., Sonozaki H., Cohen S. The production of migration inhibition factor by B and T cells of the guinea pig. J Exp Med. 1973 Oct 1;138(4):784–797. doi: 10.1084/jem.138.4.784. [DOI] [PMC free article] [PubMed] [Google Scholar]



