Abstract
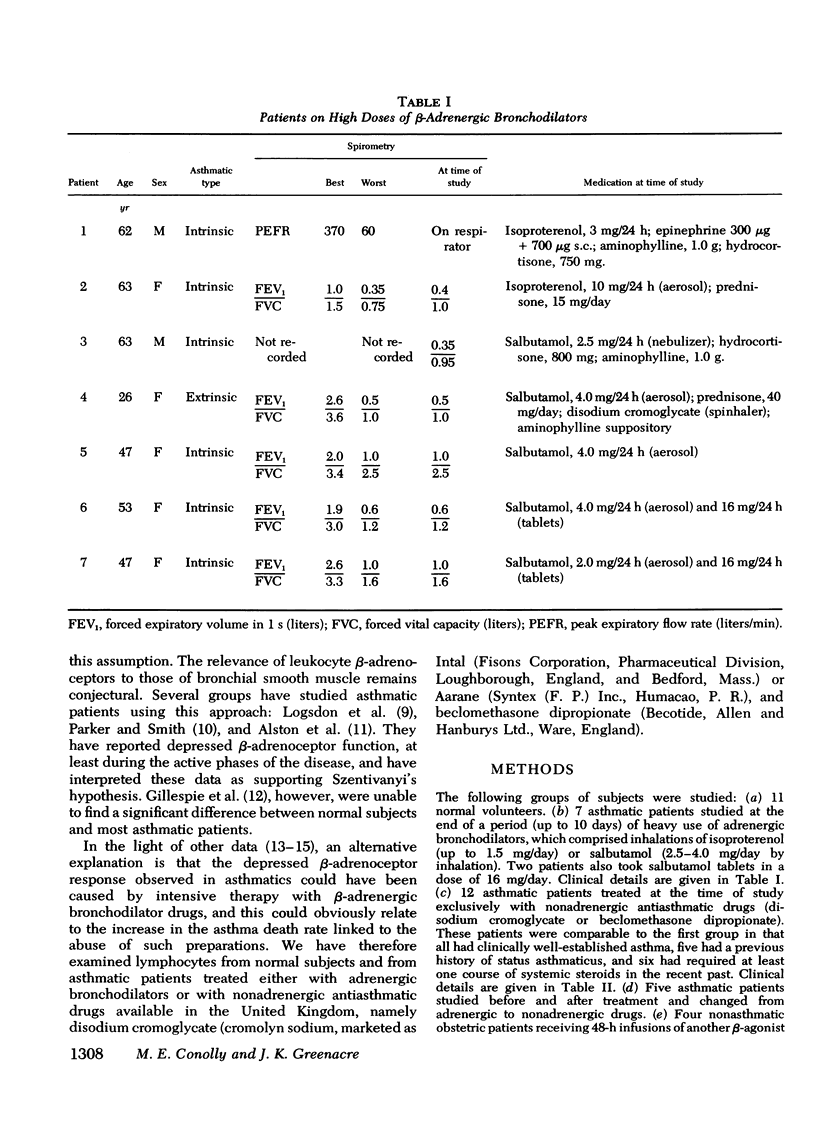
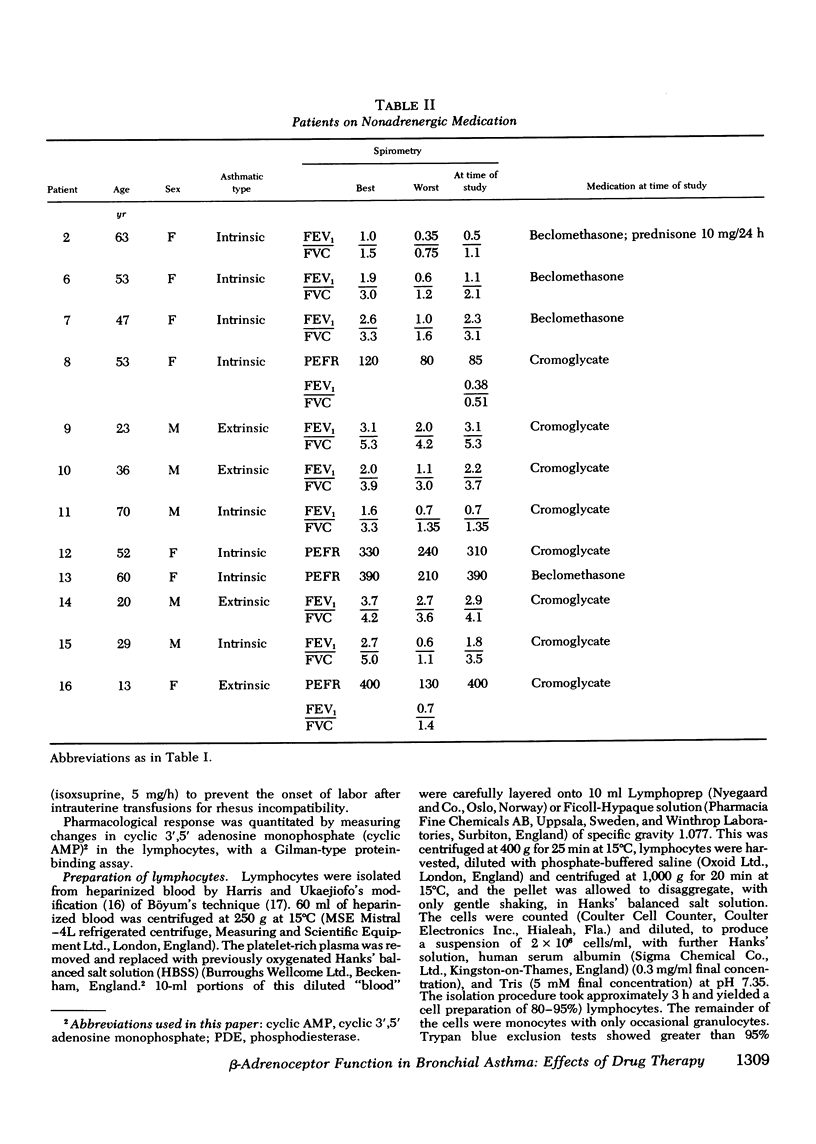
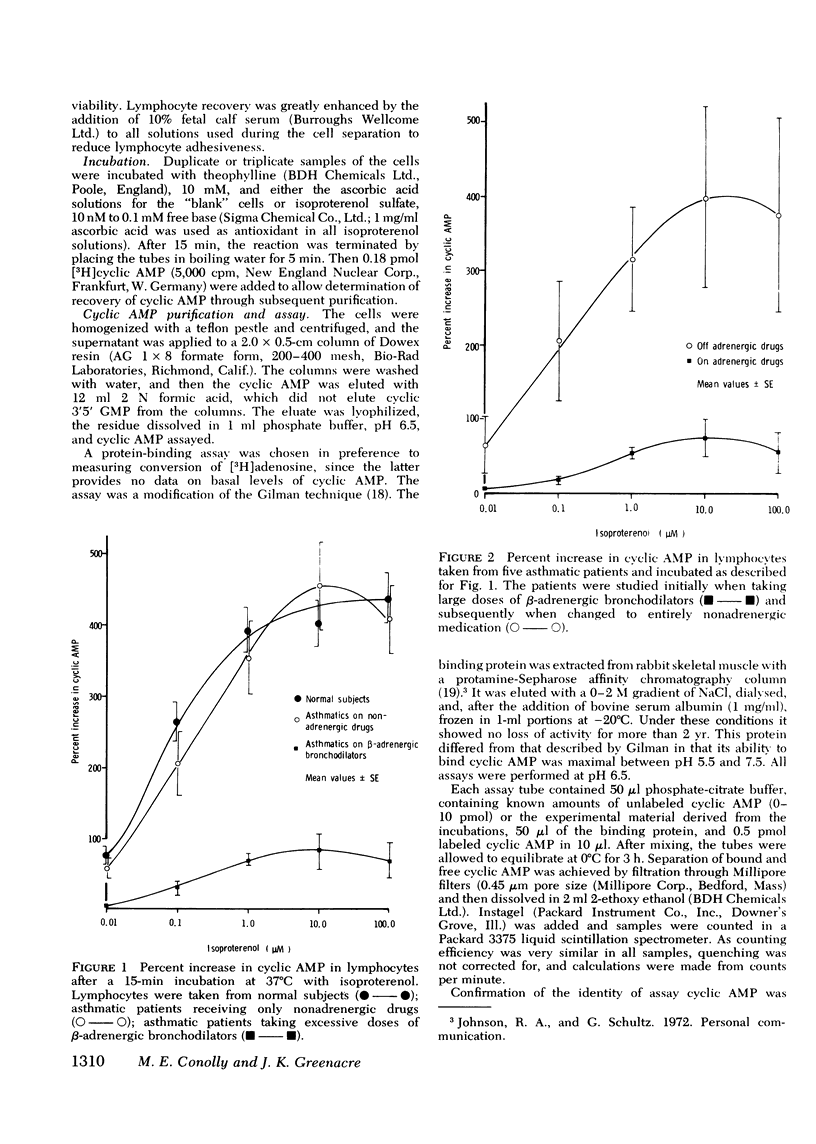
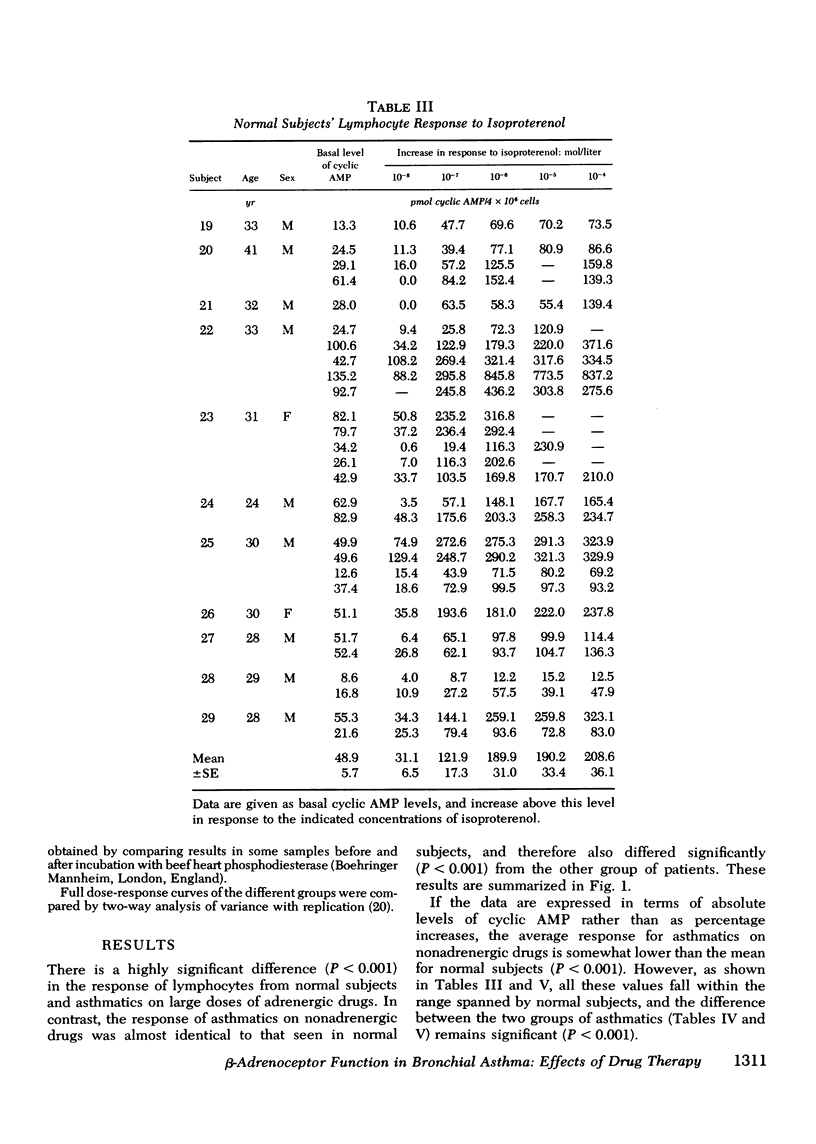
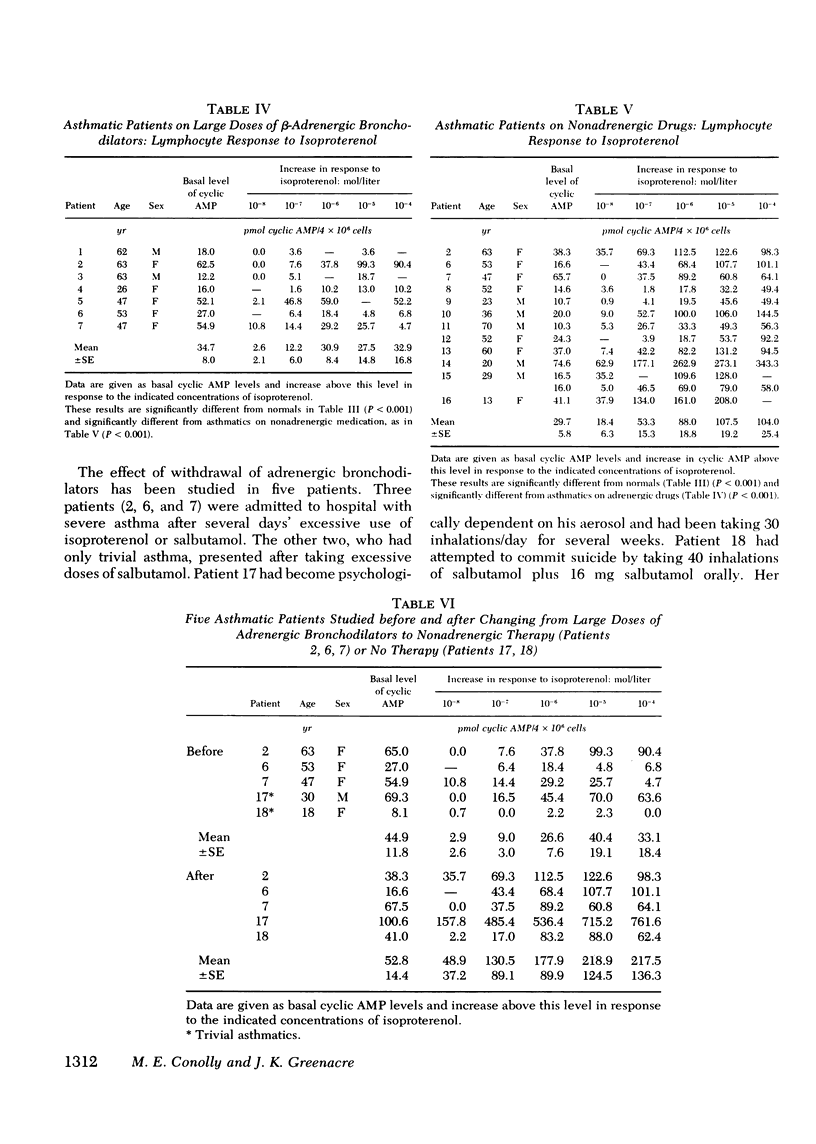
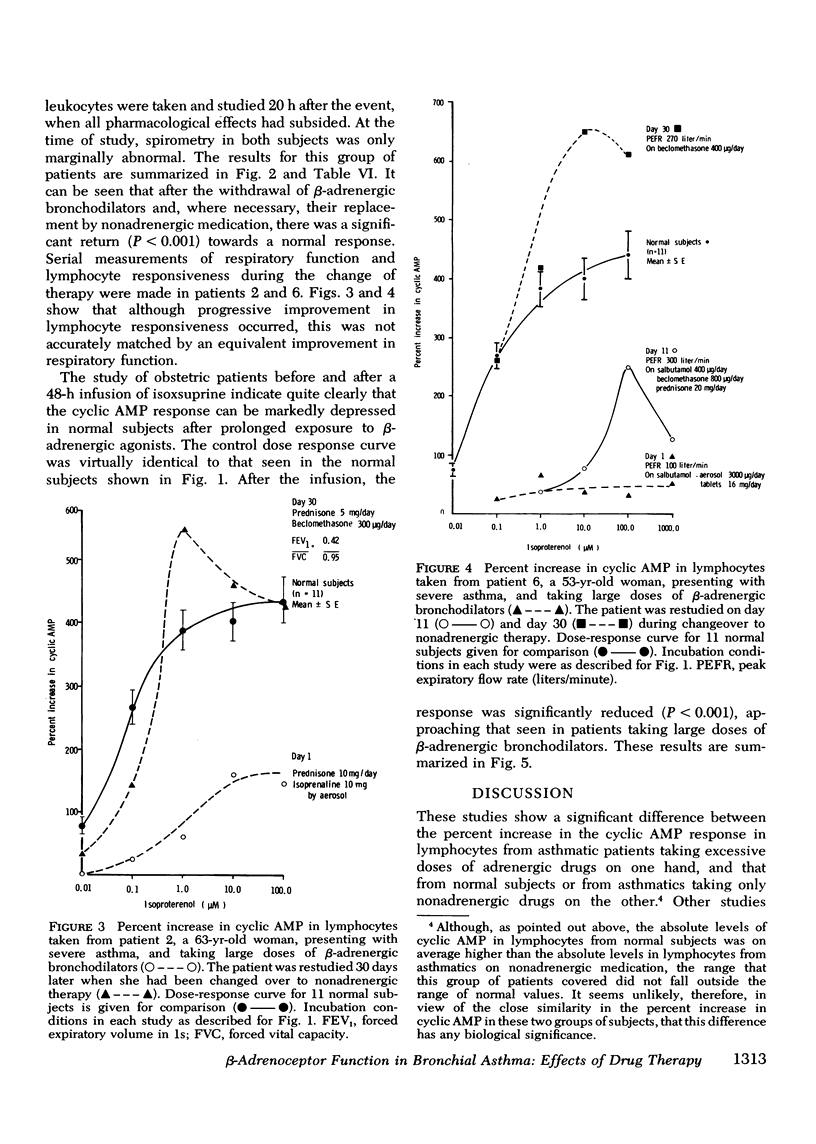
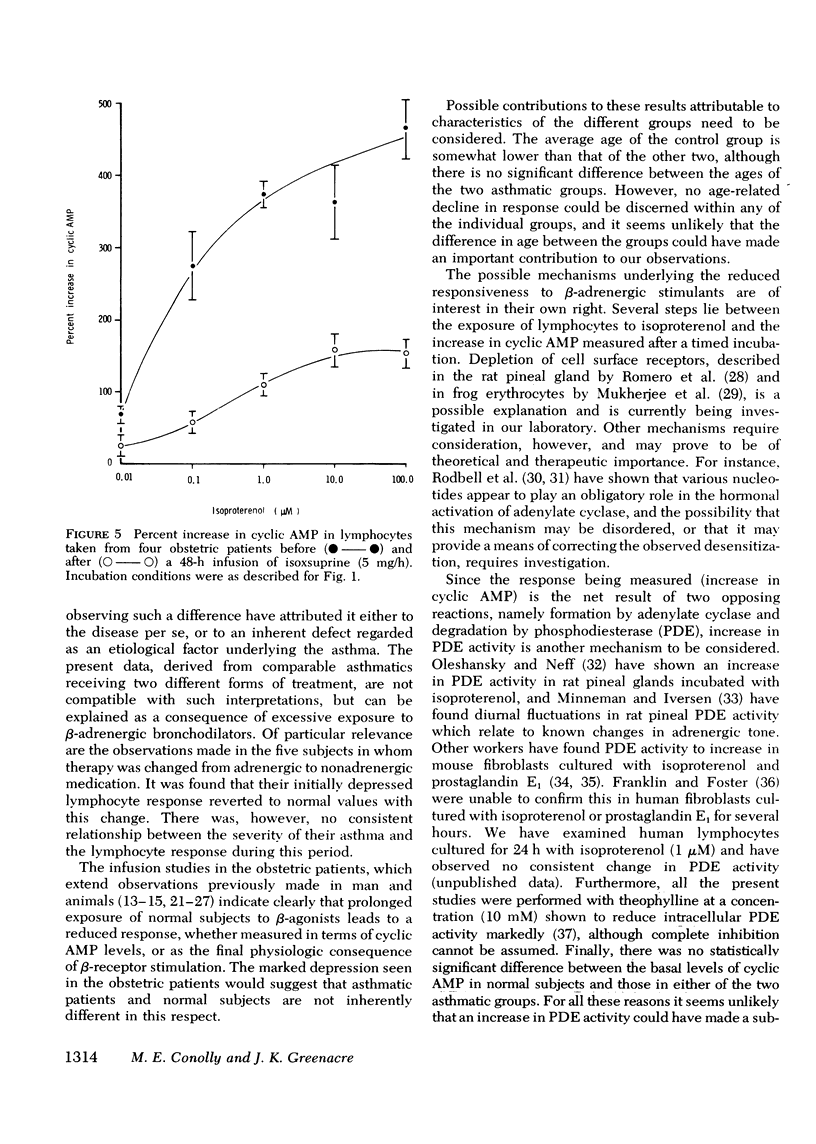
beta-Adrenoceptor function has been compared in lymphocytes of normal subjects, asthmatic patients taking large doses of beta-adrenergic bronchodilators, and comparable asthmatics treated exclusively with nonadrenergic medication. The effect of prolonged administration of beta-adrenoceptor agonists on receptor function in normal subjects has also been examined. beta-receptor response in each situation was quantitated by changes in levels of cyclic AMP, measured by a protein-binding assay. Dose response curves to isoproterenol (10 nM-0.1 mM) have been constructed for each group. Maximal increase in cyclic AMP in lymphocytes from normal subjects (393.2+/-44.0%) and in asthmatics on nonadrenergic preparations (408.3+/-46.7%) was significantly greater (P less than 0.001) than in asthmatics taking large doses of beta-sympathomimetics (67.5+/-24.2%). Depression of the cyclic AMP response appeared to correlate with the degree of exposure to beta-adrenergic agonists but not with the prevailing severity of the patient's asthma. Withdrawal of beta-adrenergic drugs was followed by a reversion of the cyclic AMP response to normal values, which suggests that the depression was drug-induced rather than an inherent feature of the disease. This interpretation was confirmed by the finding that prolonged exposure of normal subjects to high doses of a beta-adrenergic agonist caused a marked and significant (p less than 0.001) reduction in the cyclic AMP response, very similar to that seen in asthmatics on large doses of adrenergic bronchodilators. A possible link between drug-induced changes in the cyclic AMP response and the rise in the United Kingdom asthma death rate in the 1960's is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alston W. C., Patel K. R., Kerr J. W. Response of leucocyte adenyl cyclase to isoprenaline and effect of alpha-blocking drugs in extrinsic bronchial asthma. Br Med J. 1974 Jan 19;1(5898):90–93. doi: 10.1136/bmj.1.5898.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. M., Rand M. J. Mutal suppression of cardiovascular effects of some beta-adrenoreceptor agonists in the cat. J Pharm Pharmacol. 1968 Dec;20(12):916–922. doi: 10.1111/j.2042-7158.1968.tb09674.x. [DOI] [PubMed] [Google Scholar]
- BURNET F. M. MAST CELLS IN THE THYMUS OF NZB MICE. J Pathol Bacteriol. 1965 Jan;89:271–284. [PubMed] [Google Scholar]
- Bach M. A. Differences in Cyclic AMP Changes after Stimulation by Prostaglandins and Isoproterenol in Lymphocyte Subpopulations. J Clin Invest. 1975 May;55(5):1074–1081. doi: 10.1172/JCI108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoy C. J., El-Fellah M. S., Schneider R., Wade O. L. Tolerance to sympathomimetic bronchodilators in guinea-pig isolated lungs following chronic administration in vivo. Br J Pharmacol. 1975 Dec;55(4):547–554. doi: 10.1111/j.1476-5381.1975.tb07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhuys A., Douglas J. S., Lewis A. J., Eyre P. Hypersensitivity to adrenoceptor agents in the guinea-pig in vitro and in vivo. Br J Pharmacol. 1972 Nov;46(3):520P–522P. [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Lehrer R. I., Cline M. J., Melmon K. L. Cyclic 3',5'-adenosine monophosphate in the human lukocyte: synthesis, degradation, andeffects n neutrophil candidacidal activity. J Clin Invest. 1971 Apr;50(4):920–929. doi: 10.1172/JCI106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet M. Possible identification of mast cells as specialised post-mitotic cells. Med Hypotheses. 1975 Jan-Feb;1(1):3–5. doi: 10.1016/0306-9877(75)90033-x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Conolly M. E., Davies D. S., Dollery C. T., George C. F. Resistance to -adrenoceptor stimulants (a possible explanation for the rise in ashtma deaths). Br J Pharmacol. 1971 Oct;43(2):389–402. [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Armiento M., Johnson G. S., Pastan I. Regulation of adenosine 3',5'-cyclic monophosphate phosphodiesterase activity in fibroblasts by intracellular concentrations of cyclic adenosine monophosphate (3T3-dibutyryl cyclic AMP-SV40-transformed cells-michaelis constants-L cells-prostaglandin E 1 ). Proc Natl Acad Sci U S A. 1972 Feb;69(2):459–462. doi: 10.1073/pnas.69.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch J. H., Titus E. The prevention of isoproterenol desensitization and isoproterenol reversal. J Pharmacol Exp Ther. 1972 Jun;181(3):425–433. [PubMed] [Google Scholar]
- Franklin T. J., Foster S. J. Hormone-induced desensitisation of hormonal control of cyclic AMP levels in human diploid fibroblasts. Nat New Biol. 1973 Dec 5;246(153):146–148. doi: 10.1038/newbio246146a0. [DOI] [PubMed] [Google Scholar]
- Gillespie E., Valentine M. D., Lichtenstein L. M. Cyclic AMP metabolism in asthma: studies with leukocytes and lymphocytes. J Allergy Clin Immunol. 1974 Jan;53(1):27–33. doi: 10.1016/0091-6749(74)90096-7. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R., Ukaejiofo E. O. Tissue typing using a routine one-step lymphocyte separation procedure. Br J Haematol. 1970 Feb;18(2):229–235. doi: 10.1111/j.1365-2141.1970.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Hopkins S. V. Reduction in isoprenaline-induced cyclic AMP formation in guinea-pig heart after exposure to isoprenaline or salbutamol. Biochem Pharmacol. 1975 Jun 15;24(11-12):1237–1239. doi: 10.1016/0006-2952(75)90070-2. [DOI] [PubMed] [Google Scholar]
- Izard S. R., Henson E. C., Collins A. D., Brunson J. G. Increased sensitivity to anaphylactic shock in guinea pigs induced by prolonged treatment with epinephrine prior to challenge. J Allergy. 1971 Jun;47(6):309–314. doi: 10.1016/s0091-6749(71)80134-3. [DOI] [PubMed] [Google Scholar]
- Liebig R., Bernauer W., Peskar B. A. Release of prostaglandins, a prostaglandin metabolite, slow-reacting substance and histamine from anaphylactic lungs, and its modification by catecholamines. Naunyn Schmiedebergs Arch Pharmacol. 1974;284(3):279–293. doi: 10.1007/BF00500347. [DOI] [PubMed] [Google Scholar]
- Logsdon P. J., Middleton E., Jr, Coffey R. G. Stimulation of leukocyte adenyl cyclase by hydrocortisone and isoproterenol in asthmatic and nonasthmatic subjects. J Allergy Clin Immunol. 1972 Jul;50(1):45–56. doi: 10.1016/0091-6749(72)90078-4. [DOI] [PubMed] [Google Scholar]
- Maganiello V., Vaughan M. Prostaglandin E 1 effects on adenosine 3':5'-cyclic monophosphate concentration and phosphodiesterase activity in fibroblasts (mouse L cells-tissue culture-enzyme kinetics-prostaglandin homologues). Proc Natl Acad Sci U S A. 1972 Jan;69(1):269–273. doi: 10.1073/pnas.69.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneman K. P., Iversen L. L. Diurnal rhythm in rat pineal cyclic nucleotide phosphodiesterase activity. Nature. 1976 Mar 4;260(5546):59–61. doi: 10.1038/260059a0. [DOI] [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Catecholamine-induced subsensitivity of adenylate cyclase associated with loss of beta-adrenergic receptor binding sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1945–1949. doi: 10.1073/pnas.72.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleshansky M. A., Neff N. H. Rat pineal adenosine cyclic 3',5'-monophosphate phosphodiesterase activity: modulation in vivo by a beta adrenergic receptor. Mol Pharmacol. 1975 Sep;11(5):552–557. [PubMed] [Google Scholar]
- Orange R. P., Kaliner M. A., Laraia P. J., Austen K. F. Immunological release of histamine and slow reacting substance of anaphylaxis from human lung. II. Influence of cellular levels of cyclic AMP. Fed Proc. 1971 Nov-Dec;30(6):1725–1729. [PubMed] [Google Scholar]
- Parker C. W., Smith J. W. Alterations in cyclic adenosine monophosphate metabolism in human bronchial asthma. I. Leukocyte responsiveness to -adrenergic agents. J Clin Invest. 1973 Jan;52(1):48–59. doi: 10.1172/JCI107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson J. W., Conolly M. E., Davies D. S., Dollery C. T. Isoprenaline resistance and the use of pressurised aerosols in asthma. Lancet. 1968 Aug 24;2(7565):426–429. doi: 10.1016/s0140-6736(68)90467-4. [DOI] [PubMed] [Google Scholar]
- Paterson J. W., Evans R. J., Prime F. J. SElectivity of broncholidlator action of salbutamol in asthmatic patients. Br J Dis Chest. 1971 Jan;65(1):21–38. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y. Evidence for interdependent action of glucagon and nucleotides on the hepatic adenylate cyclase system. J Biol Chem. 1974 Jan 10;249(1):59–65. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y., Londos C., Harwood J. P., Martin B. R., Rendell M., Berman M. The role of adenine and guanine nucleotides in the activity and response of adenylate cyclase systems to hormones: evidence for multi-site transition states. Acta Endocrinol Suppl (Copenh) 1974;191:11–37. doi: 10.1530/acta.0.077s0011. [DOI] [PubMed] [Google Scholar]
- Romero J. A., Axelrod J. Pineal beta-adrenergic receptor: diurnal variation in sensitivity. Science. 1974 Jun 7;184(4141):1091–1092. doi: 10.1126/science.184.4141.1091. [DOI] [PubMed] [Google Scholar]
- Romero J. A., Zatz M., Kebabian J. W., Axelrod J. Circadian cycles in binding of 3H-alprenolol to beta-adrenergic receptor sites in rat pineal. Nature. 1975 Dec 4;258(5534):435–436. doi: 10.1038/258435a0. [DOI] [PubMed] [Google Scholar]
- Speizer F. E., Doll R., Heaf P. Observations on recent increase in mortality from asthma. Br Med J. 1968 Feb 10;1(5588):335–339. doi: 10.1136/bmj.1.5588.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speizer F. E., Doll R., Heaf P., Strang L. B. Investigation into use of drugs preceding death from asthma. Br Med J. 1968 Feb 10;1(5588):339–343. doi: 10.1136/bmj.1.5588.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedmyr N., Larsson S., Thiringer G. Letter: Studies of resistance to long-acting adrenergic beta-stimulators in asthmatic patients. Br Med J. 1974 Jun 22;2(5920):668–669. doi: 10.1136/bmj.2.5920.668-c. [DOI] [PMC free article] [PubMed] [Google Scholar]



