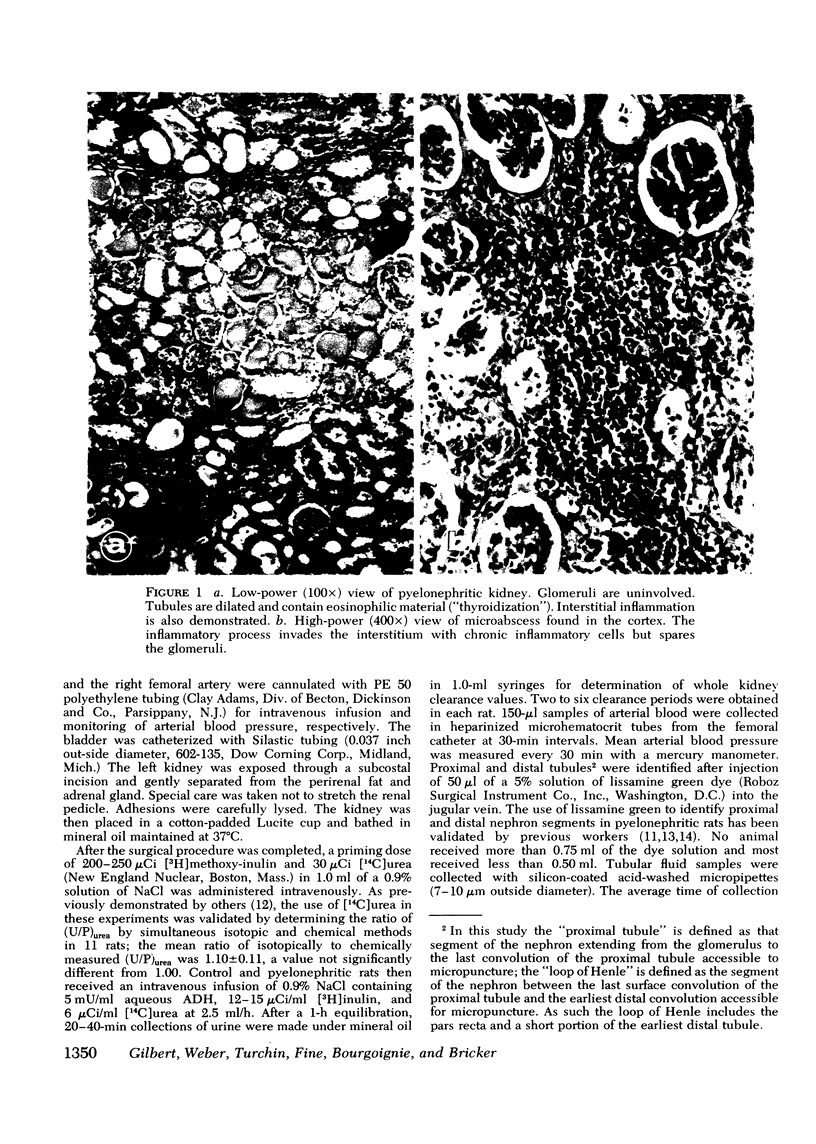
Abstract
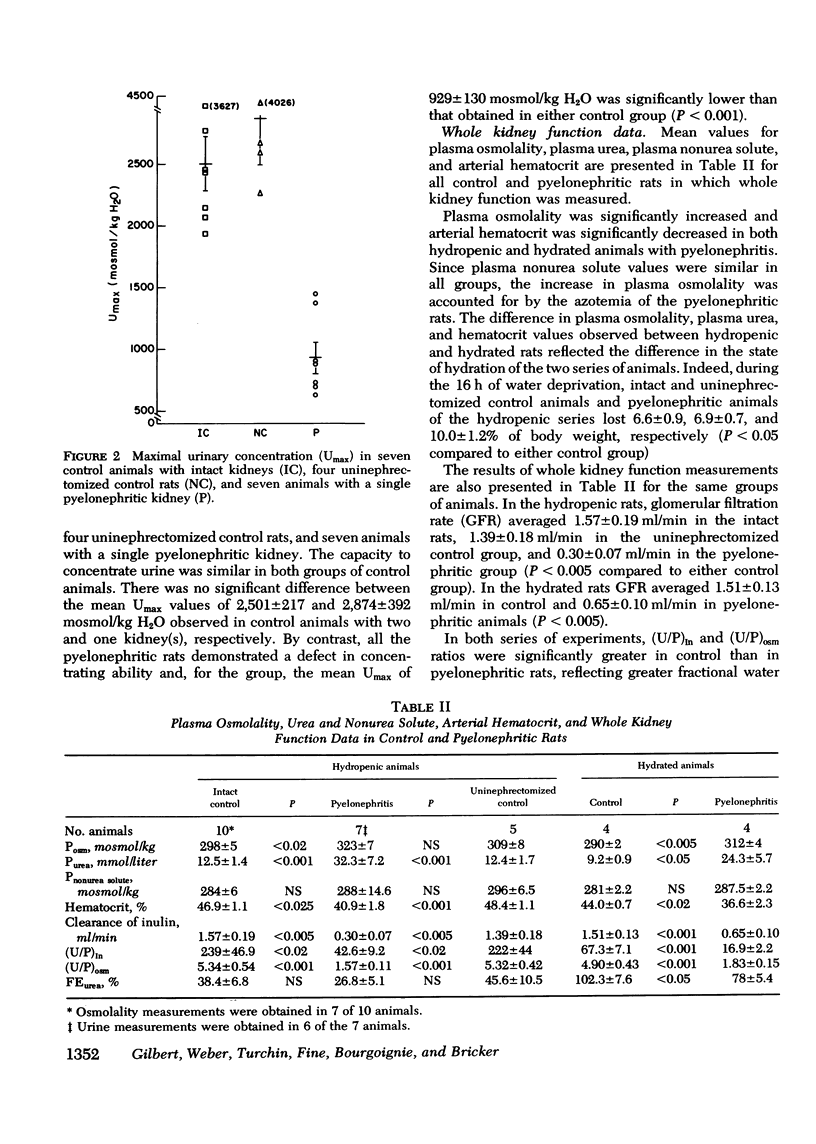
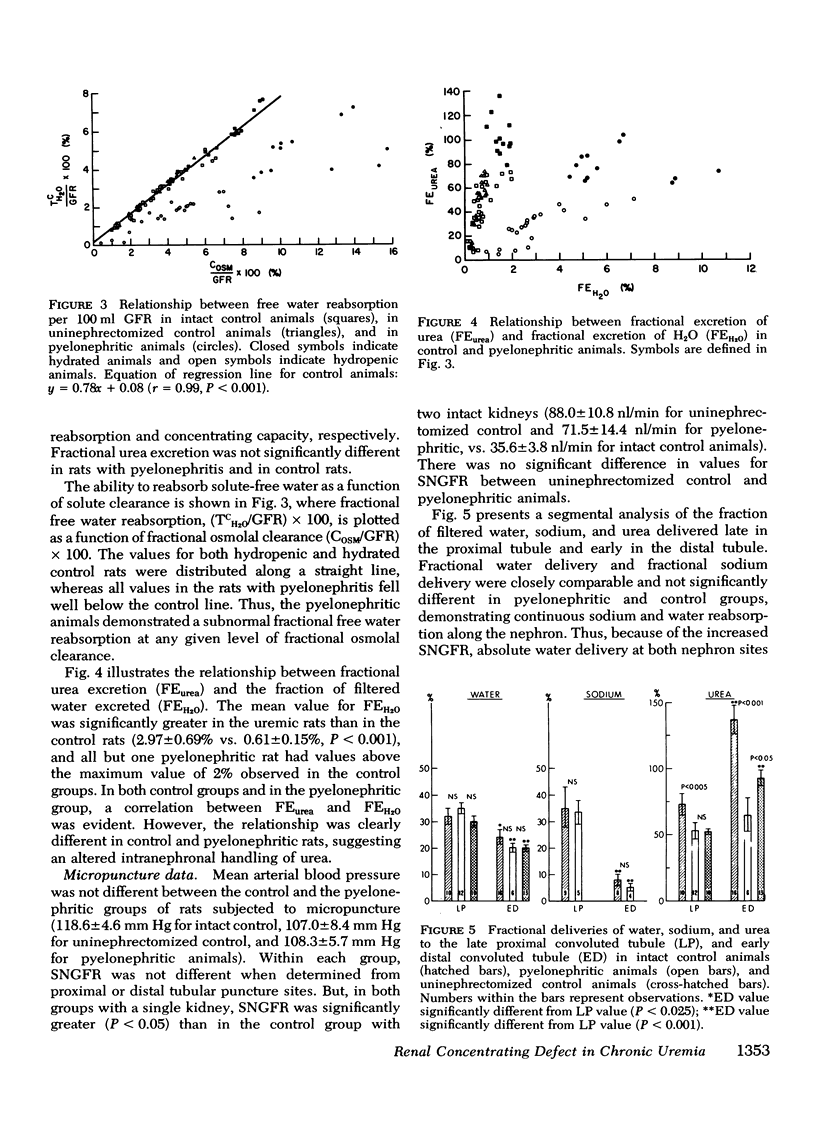
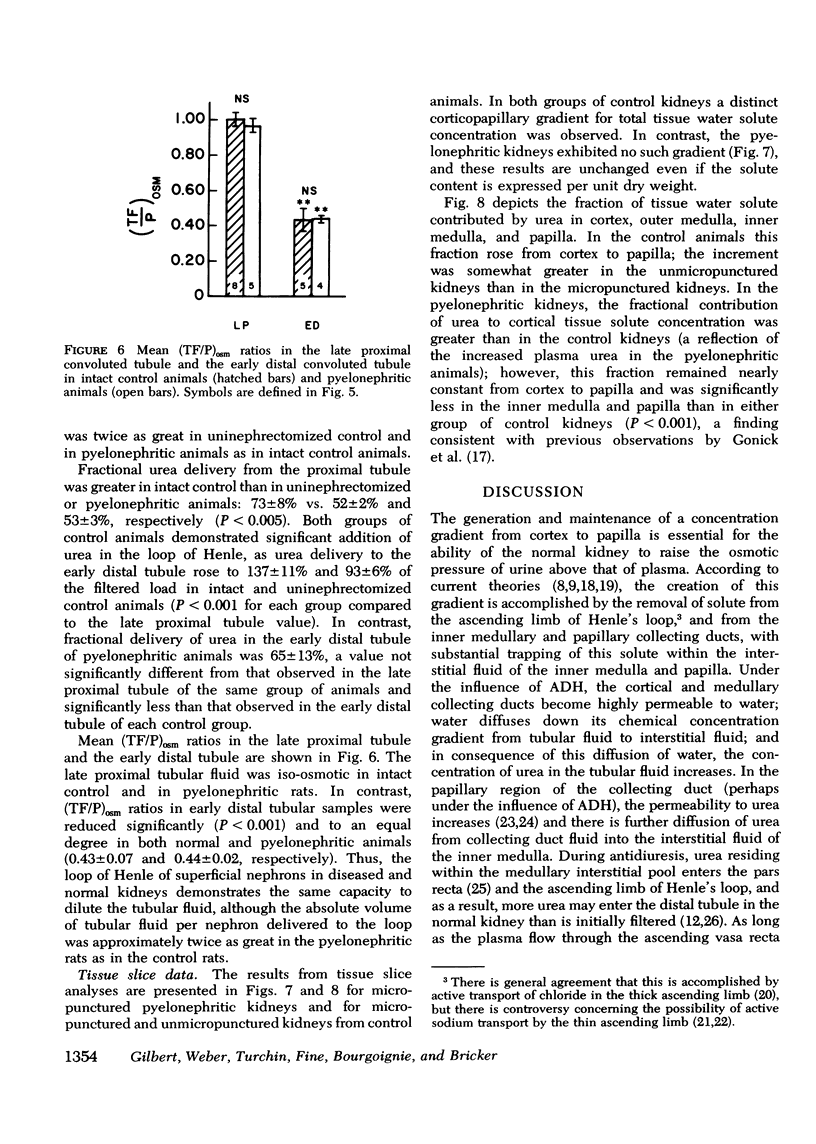
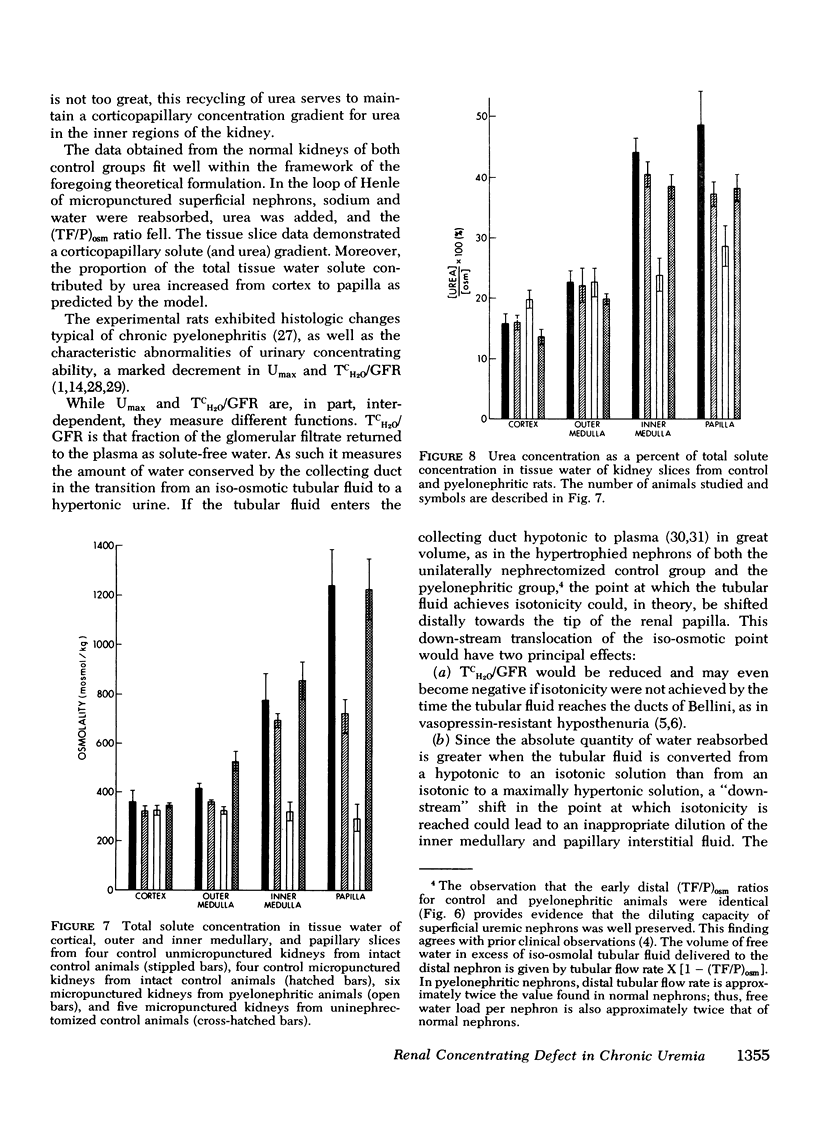
The concentrating ability of the kidney was studied by clearance and micropuncture techniques and tissue slice analyses in normal rats with two intact kidneys (intact controls), normal rats with a solitary kidney (uninephrectomized controls), and uremic rats with a single pyelonephritic kidney. Urinary osmolality after water deprivation for 24 h and administration of antidiuretic hormone was 2,501+/-217 and 2,874+/-392 mosmol/kg H2O in intact and uninephrectomized control rats, respectively, and 929+/-130 mosmol/kg H2O in pyelonephritic rats (P less than 0.001 compared to each control group). Fractional water reabsorption and concentrating ability were significantly decreased in the pyelonephritic group, and, to achieve an equivalent fractional excretion of urea, a greater fractional excretion of water was required in the pyelonephritic rats than in the control rats. Whole animal glomerular filtration rate was 1.57+/-0.19 ml/min and 1.39+/-0.18 ml/min in intact and in uninephrectomized controls, respectively, and 0.30+/-0.07 ml/min in pyelonephritic rats (P less than 0.001 compared to each control group). Single nephron glomerular filtration rate was 35.6+/-3.8 nl/min in intact control rats and was significantly increased (P less than 0.05) in both uninephrectomized (88.0+/-10.8 nl/min) and pyelonephritic rats (71.5+/-14.4 nl/min). In all groups fractional water delivery and fractional sodium delivery were closely comparable at the end of the proximal convoluted tubule and at the beginning of the distal convoluted tubule. In contrast, fractional urea delivery out of the proximal tubule was greater in the intact control group (73+/-8%) than in either the uninephrectomized (52+/-2%) or the pyelonephritic group (53+/-3%) (P less than 0.005). Fractional urea delivery at the early part of the distal tubule increased significantly to 137+/-11% and 93+/-6% of the filtered load in intact control and uninephrectomized control rats, respectively (P less than 0.001 compared to the late proximal values of each group), but failed to increase significantly in pyelonephritic rats (65+/-13%), indicating interruption of the normal recycling of urea in the latter group. Analysis of tissue slices demonstrated a rising corticopapillary gradient for total tissue water solute concentration as well as for tissue water urea concentration in both groups of control rats. In contrast, the pyelonephritic animals exhibited no similar gradients from cortex to papilla. These data indicate that the pyelonephritic kidney fails to recycle urea and accumulate interstitial solute. The latter must inevitably lead to a concentrating defect.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armsen T. R., Joppich R., Schubert G., Edel H. H. Single nephron study of intrarenal urea handling in experimental pyelonephritis. Res Exp Med (Berl) 1975 Jul 14;165(2):141–152. doi: 10.1007/BF01854840. [DOI] [PubMed] [Google Scholar]
- Armsen T., Reinhardt H. W. Transtubular movement of urea at different degrees of water diuresis. Pflugers Arch. 1971;326(3):270–280. doi: 10.1007/BF00592507. [DOI] [PubMed] [Google Scholar]
- BRICKER N. S., DEWEY R. R., LUBOWITZ H., STOKES J., KIRKENSGAARD T. Observations on the concentrating and diluting mechanisms of the diseased kidney. J Clin Invest. 1959 Mar;38(3):516–523. doi: 10.1172/JCI103829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRICKER N. S., KIME S. W., Jr, MORRIN P. A., ORLOWSKI T. The influence of glomerular filtration rate, solute excretion and hydration on the concentrating mechanism of the experimentally diseased kidney in the dog. J Clin Invest. 1960 Jun;39:864–875. doi: 10.1172/JCI104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S. Individual nephron function in experimental bilateral pyelonephritis. I. Glomerular filtration rate and proximal tubular sodium, potassium, and water reabsorption. J Lab Clin Med. 1966 Nov;68(5):713–727. [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S. Individual nephron function in experimental bilateral pyelonephritis. II. Distal tubular sodium and water reabsorption and the concentrating defect. J Lab Clin Med. 1966 Nov;68(5):728–739. [PubMed] [Google Scholar]
- Carriere S., Wong N. L., Dirks J. H. Redistribution of renal blood flow in acute and chronic reduction of renal mass. Kidney Int. 1973 Jun;3(6):364–371. doi: 10.1038/ki.1973.58. [DOI] [PubMed] [Google Scholar]
- DORHOUT MEES E. J. Role of osmotic diuresis in impairment of concentrating ability in renal disease. Br Med J. 1959 May 2;1(5130):1156–1158. doi: 10.1136/bmj.1.5130.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonick H. C., Goldberg G., Rubini M. E., Guze L. B. Functional abnormalities in experimental pyelonephritis. I. Studies of concentrating ability. Nephron. 1965;2(4):193–206. doi: 10.1159/000179403. [DOI] [PubMed] [Google Scholar]
- Gross J. B., Imai M., Kokko J. P. A functional comparison of the cortical collecting tubule and the distal convoluted tubule. J Clin Invest. 1975 Jun;55(6):1284–1294. doi: 10.1172/JCI108048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayslett J. P., Kashgarian M., Epstein F. H. Functional correlates of compensatory renal hypertrophy. J Clin Invest. 1968 Apr;47(4):774–799. doi: 10.1172/JCI105772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday M. A., Egan T. J., Morris C. R., Jarrah A. S., Harrah J. L. Pitressin-resistant hyposthenuria in chronic renal disease. Am J Med. 1967 Mar;42(3):378–387. doi: 10.1016/0002-9343(67)90266-5. [DOI] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Sodium chloride, urea, and water transport in the thin ascending limb of Henle. Generation of osmotic gradients by passive diffusion of solutes. J Clin Invest. 1974 Feb;53(2):393–402. doi: 10.1172/JCI107572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEEMAN C. R., ADAMS D. A., MAXWELL M. H. An evaluation of maximal water diuresis in chronic renal disease. I. Normal solute intake. J Lab Clin Med. 1961 Aug;58:169–184. [PubMed] [Google Scholar]
- Kawamura S., Kokko J. P. Urea secretion by the straight segment of the proximal tubule. J Clin Invest. 1976 Sep;58(3):604–612. doi: 10.1172/JCI108507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P., Rector F. C., Jr Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 1972 Oct;2(4):214–223. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- LASSITER W. E., GOTTSCHALK C. W., MYLLE M. Micropuncture study of net transtubular movement of water and urea in nondiuretic mammalian kidney. Am J Physiol. 1961 Jun;200:1139–1147. doi: 10.1152/ajplegacy.1961.200.6.1139. [DOI] [PubMed] [Google Scholar]
- Lubowitz H., Purkerson M. L., Bricker N. S. Investigation of single nephrons in the chronically diseased (Pyelonephritic) kidney of the rat using micropuncture techniques. Nephron. 1966;3(2):73–83. doi: 10.1159/000179449. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Azen S. P. Mechanism of NaCl reabsorption by hamster thin ascending limbs of Henle's loop. Am J Physiol. 1975 Jan;228(1):71–79. doi: 10.1152/ajplegacy.1975.228.1.71. [DOI] [PubMed] [Google Scholar]
- Marsh D. J. Solute and water flows in thin limbs of Henle's loop in the hamster kidney. Am J Physiol. 1970 Mar;218(3):824–831. doi: 10.1152/ajplegacy.1970.218.3.824. [DOI] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. Permeability of the loop of Henle, vasa recta, and collecting duct to water, urea, and sodium. Am J Physiol. 1968 Jul;215(1):108–115. doi: 10.1152/ajplegacy.1968.215.1.108. [DOI] [PubMed] [Google Scholar]
- Pennell J. P., Lacy F. B., Jamison R. L. An in vivo study of the concentrating process in the descending limb of Henle's loop. Kidney Int. 1974 May;5(5):337–347. doi: 10.1038/ki.1974.49. [DOI] [PubMed] [Google Scholar]
- Pennell J. P., Sanjana V., Frey N. R., Jamison R. L. The effect of urea infusion on the urinary concentrating mechanism in protein-depleted rats. J Clin Invest. 1975 Feb;55(2):399–409. doi: 10.1172/JCI107944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Permeability of medullary nephron segments to urea and water: Effect of vasopressin. Kidney Int. 1974 Dec;6(6):379–387. doi: 10.1038/ki.1974.123. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. W., Berl T. Nonosmolar factors affecting renal water excretion (second of two parts). N Engl J Med. 1975 Jan 16;292(3):141–145. doi: 10.1056/NEJM197501162920306. [DOI] [PubMed] [Google Scholar]
- Stephenson J. L. Concentration of urine in a central core model of the renal counterflow system. Kidney Int. 1972 Aug;2(2):85–94. doi: 10.1038/ki.1972.75. [DOI] [PubMed] [Google Scholar]
- Tannen R. L., Regal E. M., Dunn M. J., Schrier R. W. Vasopressin-resistant hyposthenuria in advanced chronic renal disease. N Engl J Med. 1969 May 22;280(21):1135–1141. doi: 10.1056/NEJM196905222802101. [DOI] [PubMed] [Google Scholar]
- Weiner M. W., Weinman E. J., Kashgarian M., Hayslett J. P. Accelerated reabsorption in the proximal tubule produced by volume depletion. J Clin Invest. 1971 Jul;50(7):1379–1385. doi: 10.1172/JCI106620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhall P. B., Tisher C. C. Response of the distal tubule and cortical collecting duct to vasopressin in the rat. J Clin Invest. 1973 Dec;52(12):3095–3108. doi: 10.1172/JCI107509. [DOI] [PMC free article] [PubMed] [Google Scholar]




