Abstract
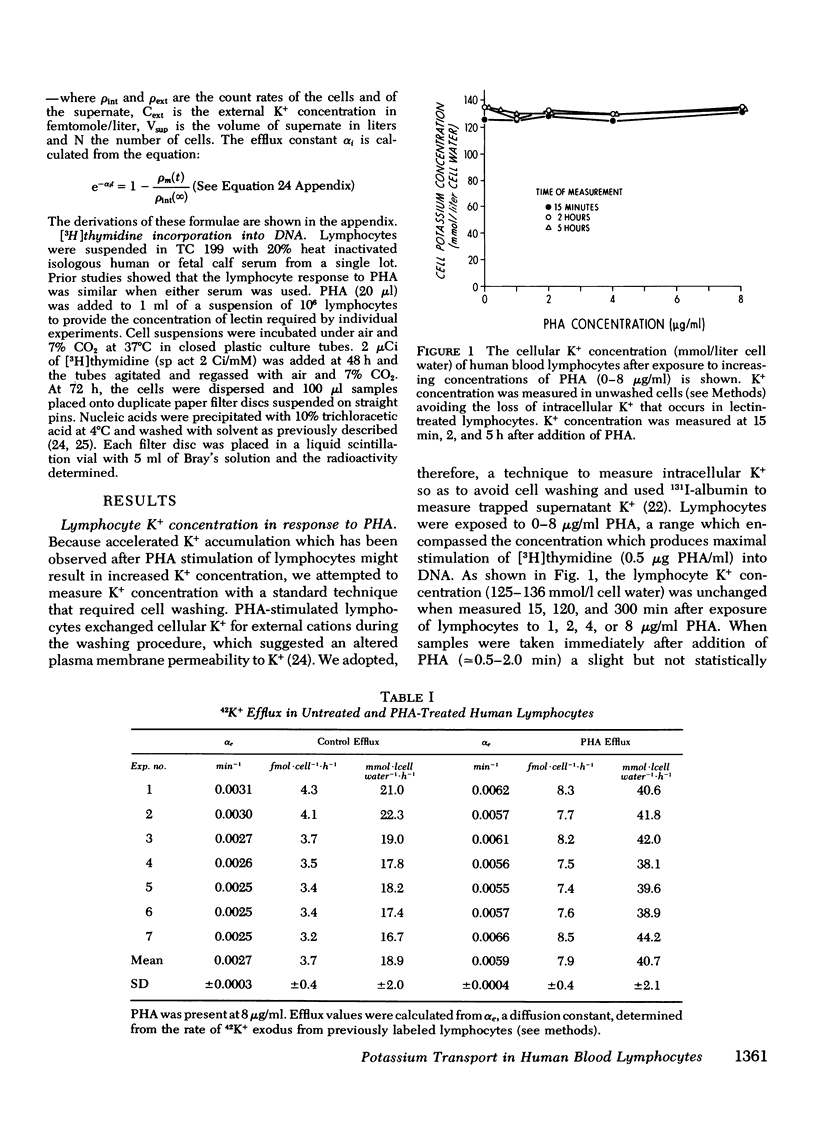
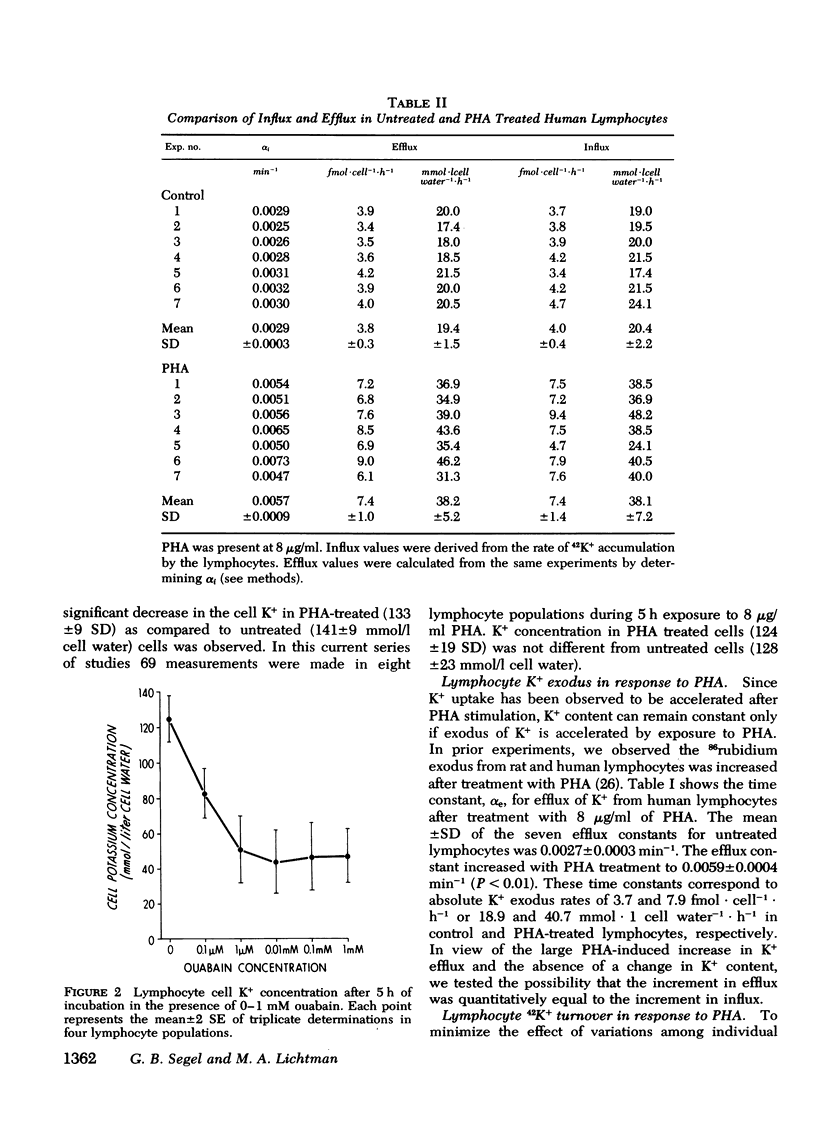
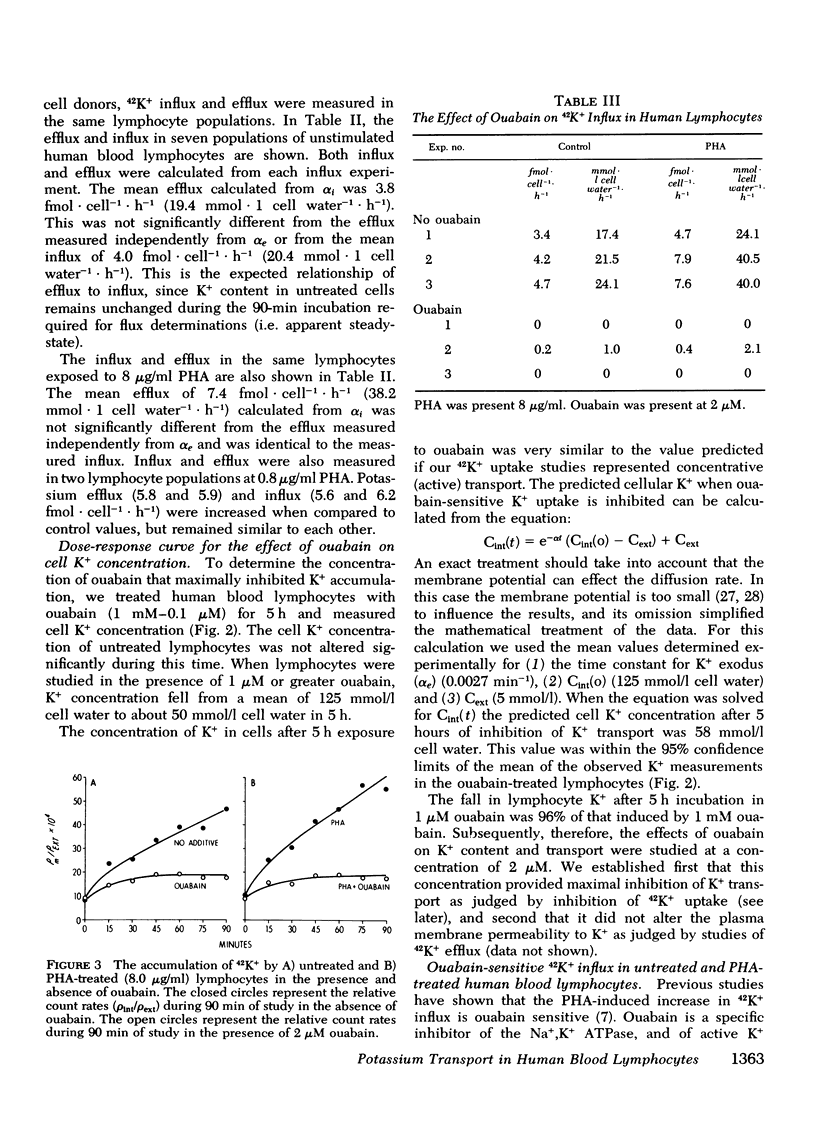
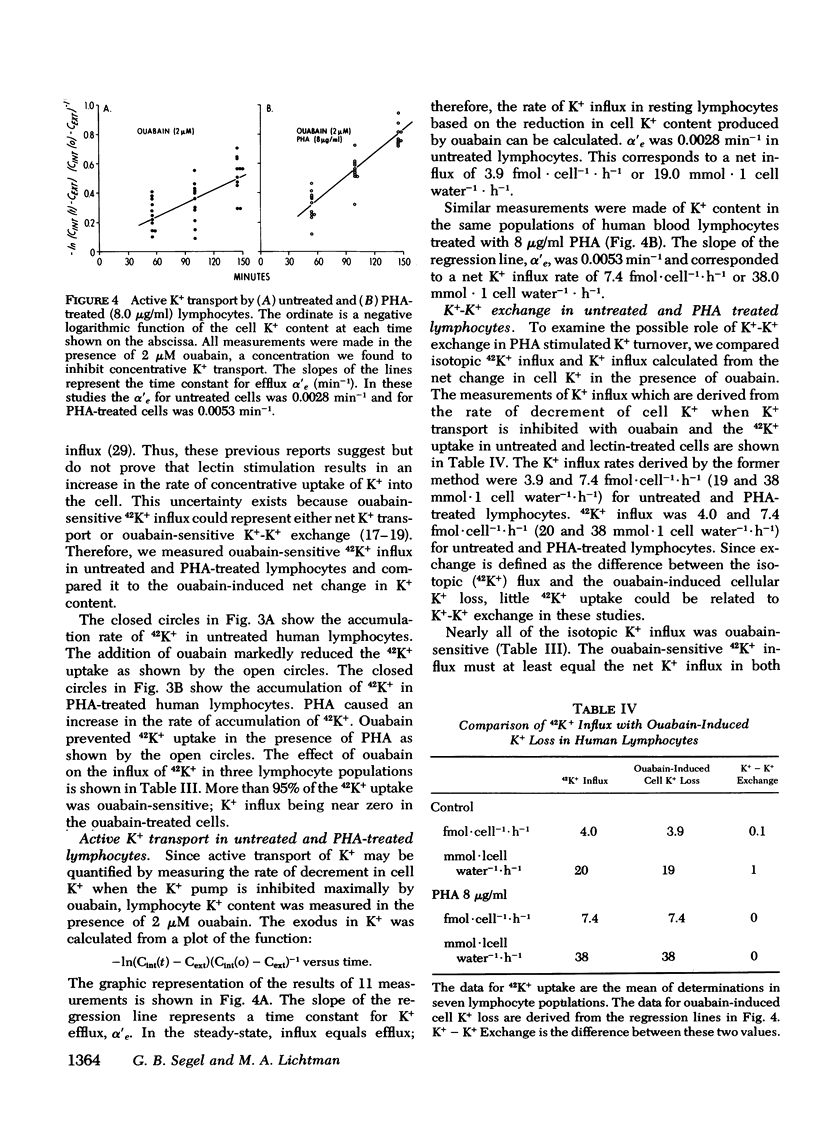
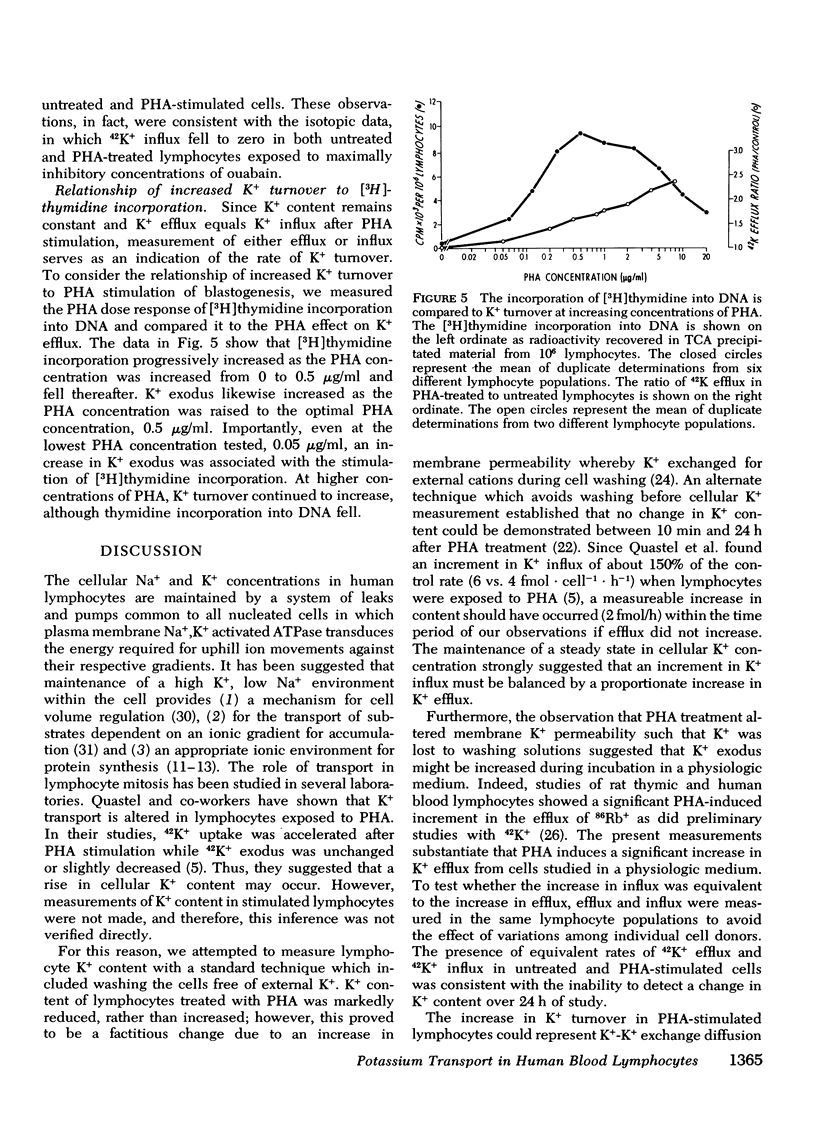
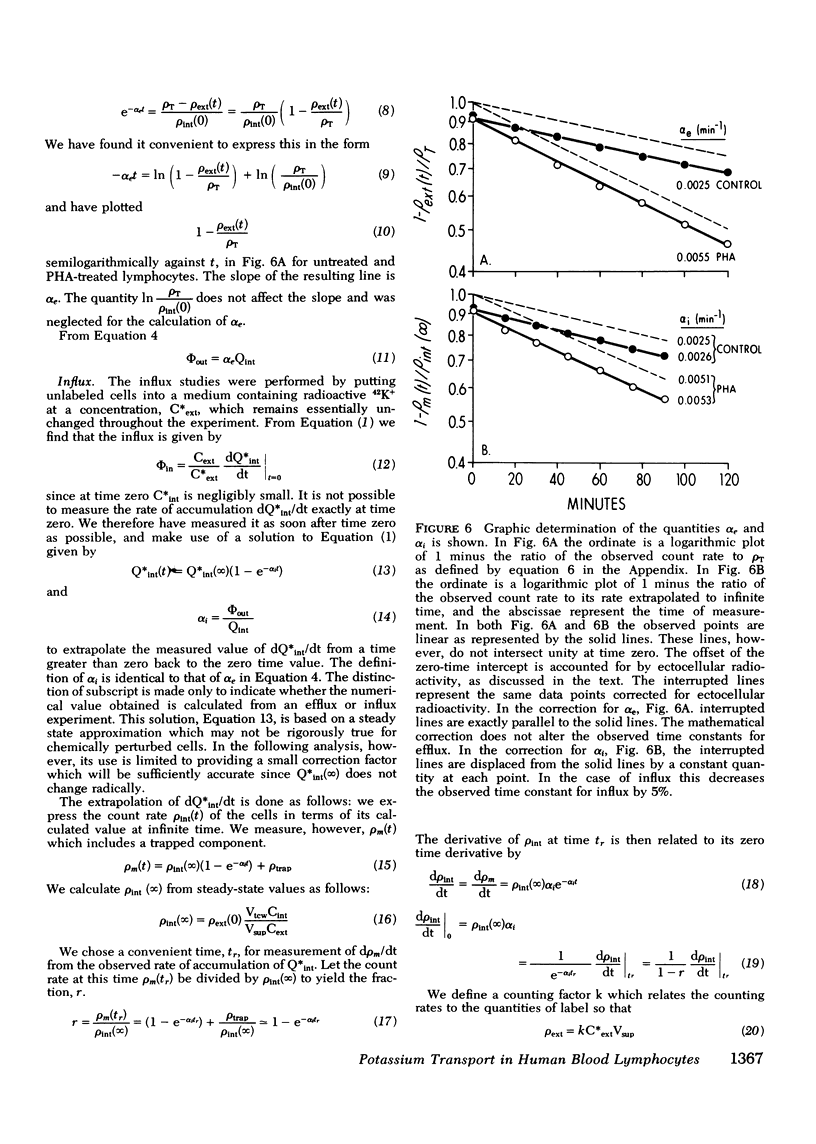
We have confirmed that phytohemagglutinin (PHA) rapidly enhances the uptake of potassium (K+) by human blood lymphocytes. PHA, however, did not produce an increase in lymphocyte K+ concentration. The apparent steady-state of cell K+ concentration despite the marked increase in uptake of 42K+ could be explained by either an increase in K+-K+ exchange or an increase in concentrative (active) K+ accumulation in association with an increase in the leak of K+ from the cell. We compared, therefore, the uptake of 42K+ with the decrement in cellular K+ content when active transport was inhibited by ouabain. These studies established that K+-K+ exchange was negligible in human blood lymphocytes and that the increase in 42K+ uptake after PHA treatment represented concentrative transport. Our studies did indicate that 42K+ exodus from PHA treated lymphocytes increased markedly from 19 to 38 mmol-1 cell water-1-h-1. Within the same time period K+ influx into PHA-treated lymphocytes increased from 20 to 38 mmol-1 cell water-1-h-1. Thus, PHA produces a marked increase in the permeability of the lymphocyte membrane to K+, and the increase in active K+ influx in PHA-treated lymphocytes may represent a homeostatic response by the membrane K+ transport system to the increase in K+ efflux. Increased K+ turnover was observed at the lowest concentrations of PHA which produced an observable increase in [3H]thymidine incorporation into DNA. Thus, PHA produces an increase in K+ permeability that closely parallels its mitogenic effect. The rapid increase in K+ influx preceding blastogenesis and mitogenesis is required, therefore, to maintain normal intracellular K+ concentration. An adequate intracellular K+ concentration is essential for the synthetic processes required for cell transformation or division.
Full text
PDF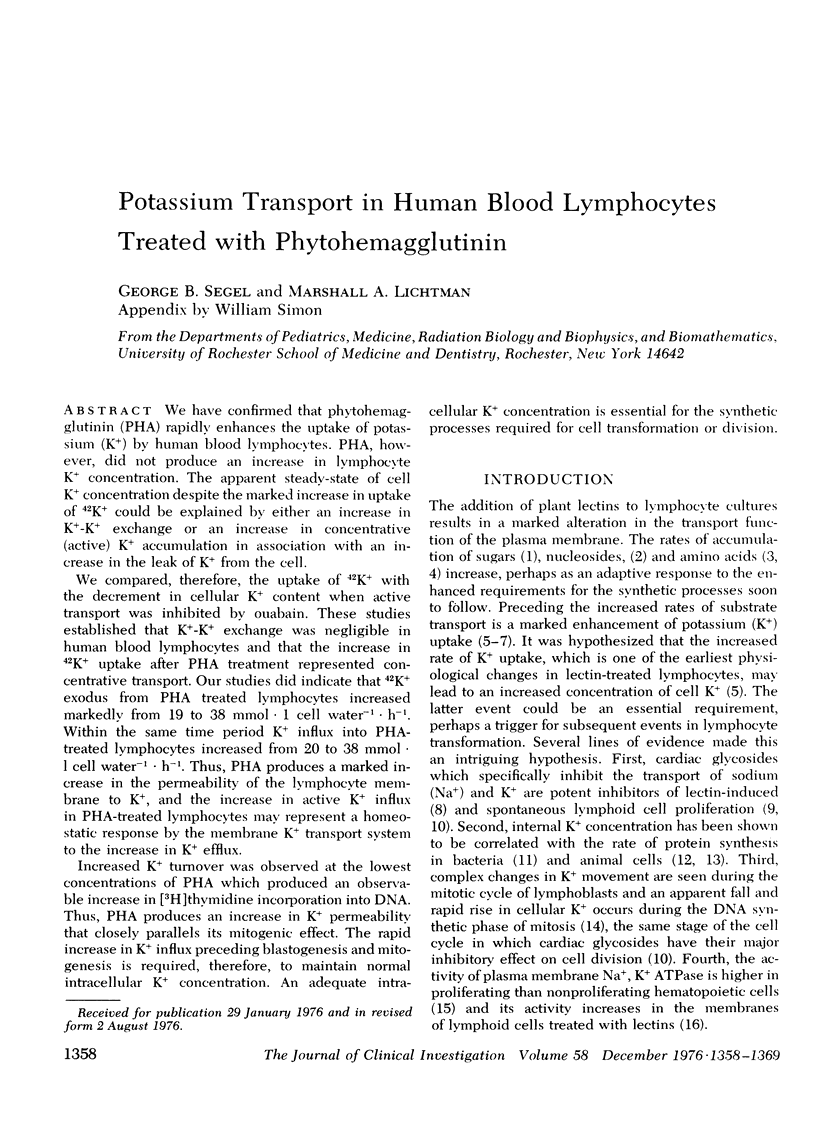





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August C. S., Merler E., Lucas D. O., Janeway C. A. The response in vitro of human lymphocytes to phytohemagglutinin and to antigens after fractionation on discontinuous density gradients of albumin. Cell Immunol. 1970 Dec;1(6):603–618. doi: 10.1016/0008-8749(70)90026-2. [DOI] [PubMed] [Google Scholar]
- Averdunk R., Lauf P. K. Effects of mitogens on sodium-potassium transport, 3H-ouabain binding, and adenosine triphosphatase activity in lymphocytes. Exp Cell Res. 1975 Jul;93(2):331–342. doi: 10.1016/0014-4827(75)90458-9. [DOI] [PubMed] [Google Scholar]
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Cuff J. M., Lichtman A. The early effects of ouabain on potassium metabolism and rate of proliferation of mouse lymphoblasts. J Cell Physiol. 1975 Apr;85(2 Pt 1):209–215. doi: 10.1002/jcp.1040850207. [DOI] [PubMed] [Google Scholar]
- Cuff J. M., Lichtman M. A. The effects of ouabain on the cell mitotic cycle of mouse lymphoblasts. J Cell Physiol. 1975 Apr;85(2 Pt 1):227–234. doi: 10.1002/jcp.1040850209. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M. Membrane adenosine triphosphatase and cation transport. Br Med Bull. 1968 May;24(2):165–169. doi: 10.1093/oxfordjournals.bmb.a070620. [DOI] [PubMed] [Google Scholar]
- Jung C., Rothstein A. Cation metabolism in relation to cell size in synchronously grown tissue culture cell. J Gen Physiol. 1967 Mar;50(4):917–932. doi: 10.1085/jgp.50.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBIN M. INTRACELLULAR POTASSIUM AND CONTROL OF PROTEIN SYNTHESIS. Fed Proc. 1964 Sep-Oct;23:994–1001. [PubMed] [Google Scholar]
- Lauf P. K. Antigen-antibody reactions and cation transport in biomembranes: immunophysiological aspects. Biochim Biophys Acta. 1975 Jun 30;415(2):173–229. doi: 10.1016/0304-4157(75)90002-7. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Jackson A. H., Peck W. A. Lymphocyte monovalent cation metabolism: cell volume, cation content and cation transport. J Cell Physiol. 1972 Dec;80(3):383–396. doi: 10.1002/jcp.1040800309. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. The monovalent cation content and adenosine triphosphatase activity of human normal and leukemic granulocytes and lymphocytes: relationship to cell volume and morphologic age. Blood. 1969 Nov;34(5):645–660. [PubMed] [Google Scholar]
- Lubin M. Intracellular potassium and macromolecular synthesis in mammalian cells. Nature. 1967 Feb 4;213(5075):451–453. doi: 10.1038/213451a0. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Skinner A., Kornfeld S. The rapid induction by phytohemagglutinin of increased alpha-aminoisobutyric acid uptake by lymphocytes. J Clin Invest. 1971 Apr;50(4):818–826. doi: 10.1172/JCI106553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. H., Hausen P. Effect of phytohemagglutinin on lymphocyte membrane transport. 2. Stimulation of "facilitated diffusion" of 3-O-methyl-glucose. Eur J Biochem. 1971 Apr 30;19(4):509–513. doi: 10.1111/j.1432-1033.1971.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Hausen P. Effect of phytohemagglutinin on lymphocyte membrane transport. I. Stimulation of uridine uptake. Eur J Biochem. 1971 Apr 30;19(4):502–508. doi: 10.1111/j.1432-1033.1971.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Quastel M. R., Kaplan J. G. Early stimulation of potassium uptake in lymphocytes treated with PHA. Exp Cell Res. 1970 Nov;63(1):230–233. doi: 10.1016/0014-4827(70)90360-5. [DOI] [PubMed] [Google Scholar]
- Quastel M. R., Kaplan J. G. Inhibition by ouabain of human lymphocyte transformation induced by phytohaemagglutinin in vitro. Nature. 1968 Jul 13;219(5150):198–200. doi: 10.1038/219198a0. [DOI] [PubMed] [Google Scholar]
- Read C. P. Studies on membrane transport. I. A common transport system for sugars and amino acids. Biol Bull. 1967 Dec;133(3):630–642. doi: 10.2307/1539924. [DOI] [PubMed] [Google Scholar]
- Sachs J. R. Recoupling the Na-K pump. J Clin Invest. 1972 Dec;51(12):3244–3247. doi: 10.1172/JCI107151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel G. B., Feig S. A., Glader B. E., Muller A., Dutcher P., Nathan D. G. Energy metabolism in human erythrocytes: the role of phosphoglycerate kinase in cation transport. Blood. 1975 Aug;46(2):271–278. [PubMed] [Google Scholar]
- Segel G. B., Gordon B. R., Lichtman M. A., Hollander M. M., Klemperer M. R. Exodus of 42K+ and 86Rb+ from rat thymic and human blood lymphocytes exposed to phytohemagglutinin. J Cell Physiol. 1976 Mar;87(3):337–343. doi: 10.1002/jcp.1040870309. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Hollander M. M., Gordon B. R., Klemperer M. R., Lichtman M. A. A rapid phytohemagglutinin induced alteration in lymphocyte potassium permeability. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):327–335. doi: 10.1002/jcp.1040860404. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A., Gordon B. R., MacPherson J. L., Nusbacher J. Plateletpheresis residues: a source of large quantities of human blood lymphocytes. Transfusion. 1976 Sep-Oct;16(5):455–459. doi: 10.1046/j.1537-2995.1976.16577039302.x. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A., Hollander M. M., Gordon B. R., Klemperer M. R. Human lymphocyte potassium content during the initiation of phytohemagglutinin-induced mitogenesis. J Cell Physiol. 1976 May;88(1):43–48. doi: 10.1002/jcp.1040880106. [DOI] [PubMed] [Google Scholar]
- Taki M. Studies on blastogenesis of human lymphocytes by phytohemagglutinin, with special reference to changes of membrane potential during blastoid transformation. Mie Med J. 1970 Jan;19(3):245–262. [PubMed] [Google Scholar]
- van den Berg K. J., Betel I. Early increase of amino acid transport in stimulated lymphocytes. Exp Cell Res. 1971 May;66(1):257–259. doi: 10.1016/s0014-4827(71)80037-x. [DOI] [PubMed] [Google Scholar]



