Abstract
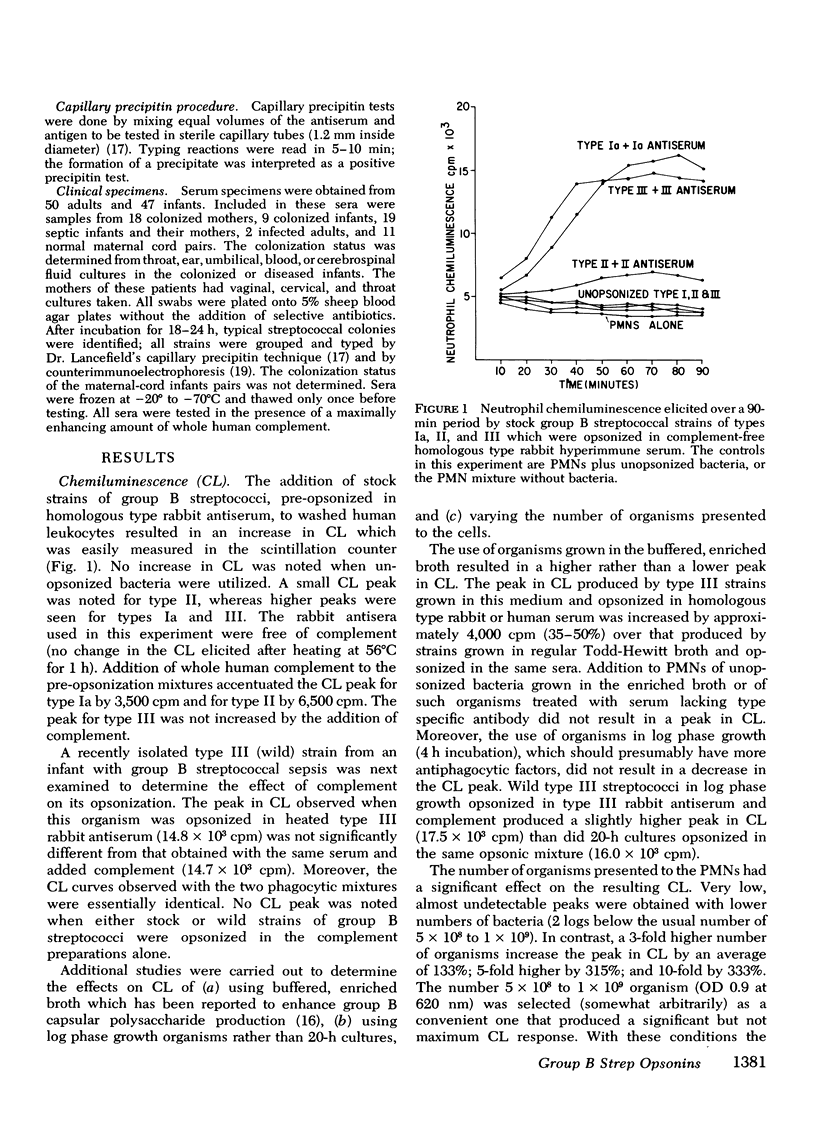
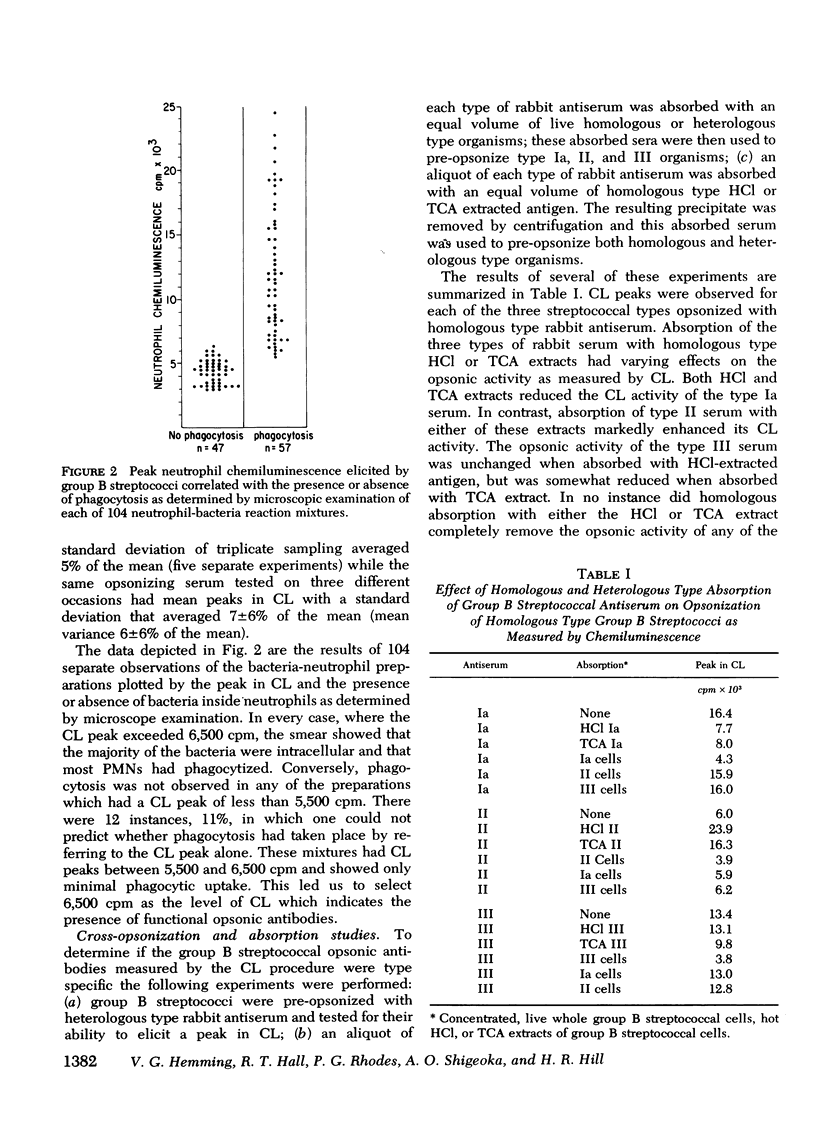
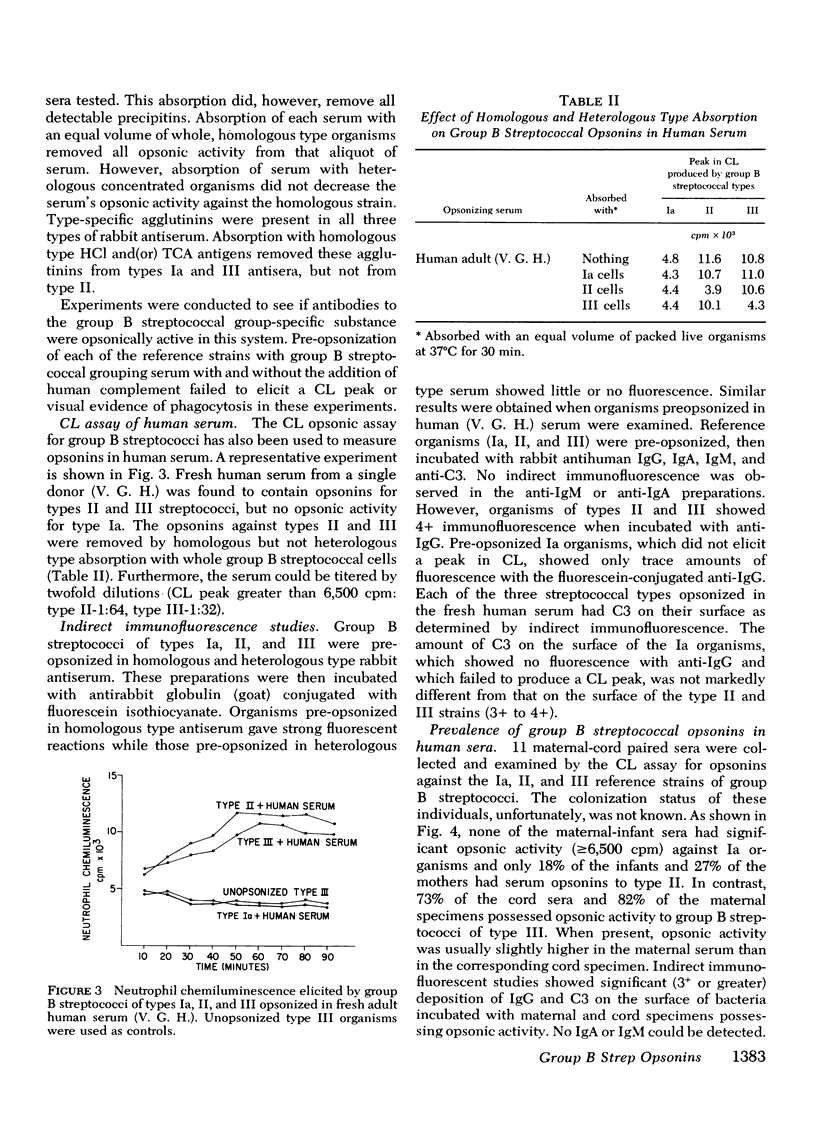
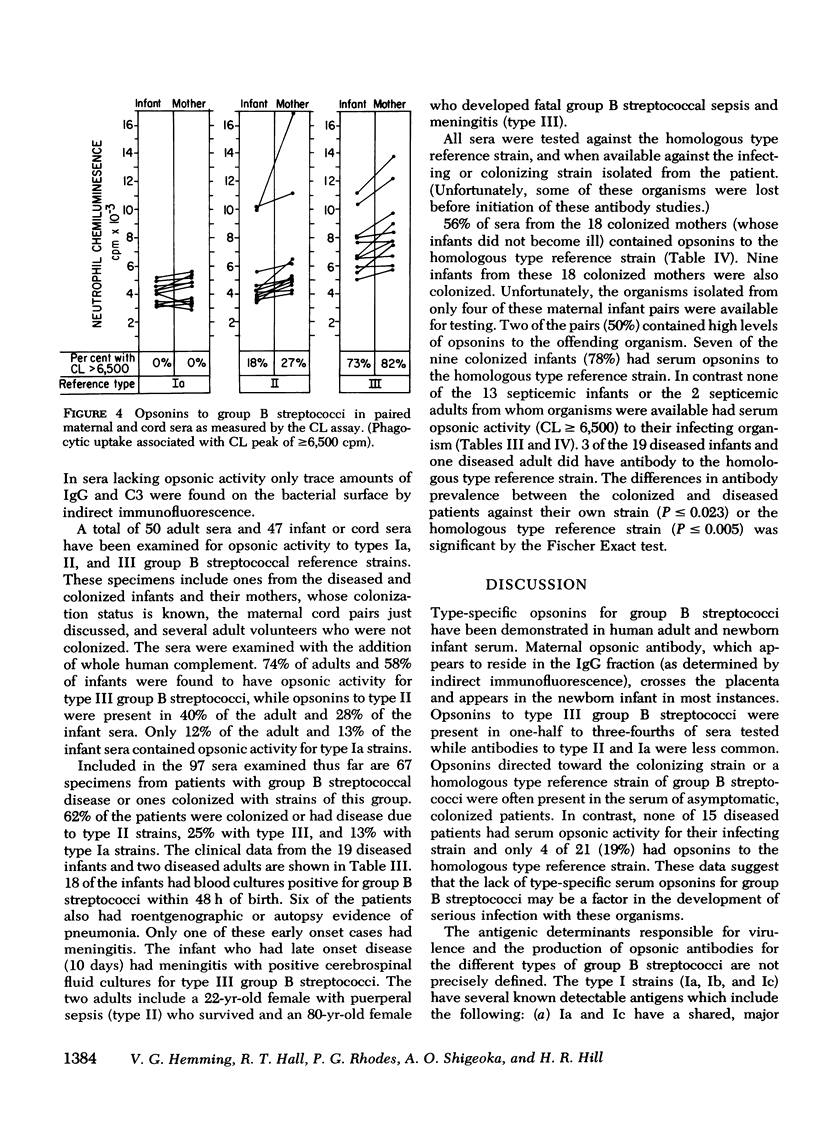
The factors important in host defense against group B streptococci are not well understood. The role of antibody and complement in the prevention of serious infection by these organisms is not known because, to date, a reliable measure of functional opsonic activity has not been developed. Recently, it has been shown that neutrophils produce a chemiluminescence after ingestion of particulate matter, and that this event can be detected and quantitated in a liquid scintillation system. We have adapted the chemiluminescence procedure to examine rabbit hyperimmune and human serum for the presence of group B streptococcal opsonins. Group B streptococci of types Ia, II, and III that were opsonized in homologous but not heterologous type serum produced a peak in chemiluminescence when added to normal human neutrophils. Such activity was correlated, in each instance, with ingestion of bacteria by neutrophils and deposition of immunoglobulin and C3 on the bacterial surface as detected by indirect immunofluorescence. With this assay, we have examined sera from colonized and diseased patients for the presence of opsonins to types Ia, II, and III group B streptococci. Maternal sera often contained type-specific opsonins which resided in the IgG fraction and which crossed the placenta to appear in paired cord specimens. 63% of patients colonized with group B streptococci had serum opsonins to their colonizing type of organism. In contrast, none of the 15 patients with sepsis or meningitis had opsonins directed against their infecting strain. These data suggest that the lack of type-specific opsonins to group B streptococci may be one of the important factors in determining host susceptibility to systemic infection with strains of this group.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Halide dependence of the myeloperoxidase-mediated antimicrobial system of the polymorphonuclear leukocyte in the phenomenon of electronic excitation. Biochem Biophys Res Commun. 1975 Apr 7;63(3):675–683. doi: 10.1016/s0006-291x(75)80437-2. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Allen R. C. The role of PH in the chemiluminescent response of the myeloperoxidase-halide-HOOH antimicrobial system. Biochem Biophys Res Commun. 1975 Apr 7;63(3):684–691. doi: 10.1016/s0006-291x(75)80438-4. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Yevich S. J., Orth R. W., Steele R. H. The superoxide anion and singlet molecular oxygen: their role in the microbicidal activity of the polymorphonuclear leukocyte. Biochem Biophys Res Commun. 1974 Oct 8;60(3):909–917. doi: 10.1016/0006-291x(74)90401-x. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Barrett F. F., Gordon R. C., Yow M. D. Suppurative meningitis due to streptococci of Lancefield group B: a study of 33 infants. J Pediatr. 1973 Apr;82(4):724–729. doi: 10.1016/s0022-3476(73)80606-7. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Barrett F. F. Transmission of group B streptococci among parturient women and their neonates. J Pediatr. 1973 Dec;83(6):919–925. doi: 10.1016/s0022-3476(73)80524-4. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976 Apr 1;294(14):753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Microcapsule of type III strains of group B Streptococcus: production and morphology. Infect Immun. 1976 Jan;13(1):189–194. doi: 10.1128/iai.13.1.189-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossett J. H., Williams R. C., Jr, Quie P. G. Studies on interaction of bacteria, serum factors and polymorphonuclear leukocytes in mothers and newborns. Pediatrics. 1969 Jul;44(1):49–57. [PubMed] [Google Scholar]
- EICKHOFF T. C., KLEIN J. O., DALY A. K., INGALL D., FINLAND M. NEONATAL SEPSIS AND OTHER INFECTIONS DUE TO GROUP B BETA-HEMOLYTIC STREPTOCOCCI. N Engl J Med. 1964 Dec 10;271:1221–1228. doi: 10.1056/NEJM196412102712401. [DOI] [PubMed] [Google Scholar]
- Franciosi R. A., Knostman J. D., Zimmerman R. A. Group B streptococcal neonatal and infant infections. J Pediatr. 1973 Apr;82(4):707–718. doi: 10.1016/s0022-3476(73)80604-3. [DOI] [PubMed] [Google Scholar]
- HOOD M., JANNEY A., DAMERON G. Beta hemolytic streptococcus group B associated with problems of the perinatal period. Am J Obstet Gynecol. 1961 Oct;82:809–818. doi: 10.1016/s0002-9378(16)36146-4. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Riter M. E., Menge S. K., Johnson D. R., Matsen J. M. Rapid identification of group B streptococci by counterimmunoelectrophoresis. J Clin Microbiol. 1975 Feb;1(2):188–191. doi: 10.1128/jcm.1.2.188-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. B., McCracken G. H., Jr The spectrum of group B streptococcal infections in infancy. Am J Dis Child. 1974 Dec;128(6):815–818. doi: 10.1001/archpedi.1974.02110310063011. [DOI] [PubMed] [Google Scholar]
- Klesius P. H., Zimmerman R. A., Mathews J. H., Krushak D. H. Cellular and humoral immune response to group B streptococci. J Pediatr. 1973 Dec;83(6):926–932. doi: 10.1016/s0022-3476(73)80525-6. [DOI] [PubMed] [Google Scholar]
- Lancefield R. C., Freimer E. H. Type-specific polysaccharide antigens of group B streptococci. J Hyg (Lond) 1966 Jun;64(2):191–203. doi: 10.1017/s0022172400040456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancefield R. C., McCarty M., Everly W. N. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975 Jul 1;142(1):165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews J. H., Klesius P. H., Zimmerman R. A. Opsonin system of the group B streptococcus. Infect Immun. 1974 Dec;10(6):1315–1320. doi: 10.1128/iai.10.6.1315-1320.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjernholm R. L., Allen R. C., Steele R. H., Waring W. W., Harris J. A. Impaired chemiluminescence during phagocytosis of opsonized bacteria. Infect Immun. 1973 Feb;7(2):313–314. doi: 10.1128/iai.7.2.313-314.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H. W., Facklam R. R., Wortham E. C. Distribution by serological type of group B streptococci isolated from a variety of clinical material over a five-year period (with special reference to neonatal sepsis and meningitis). Infect Immun. 1973 Aug;8(2):228–235. doi: 10.1128/iai.8.2.228-235.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



