Abstract
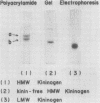
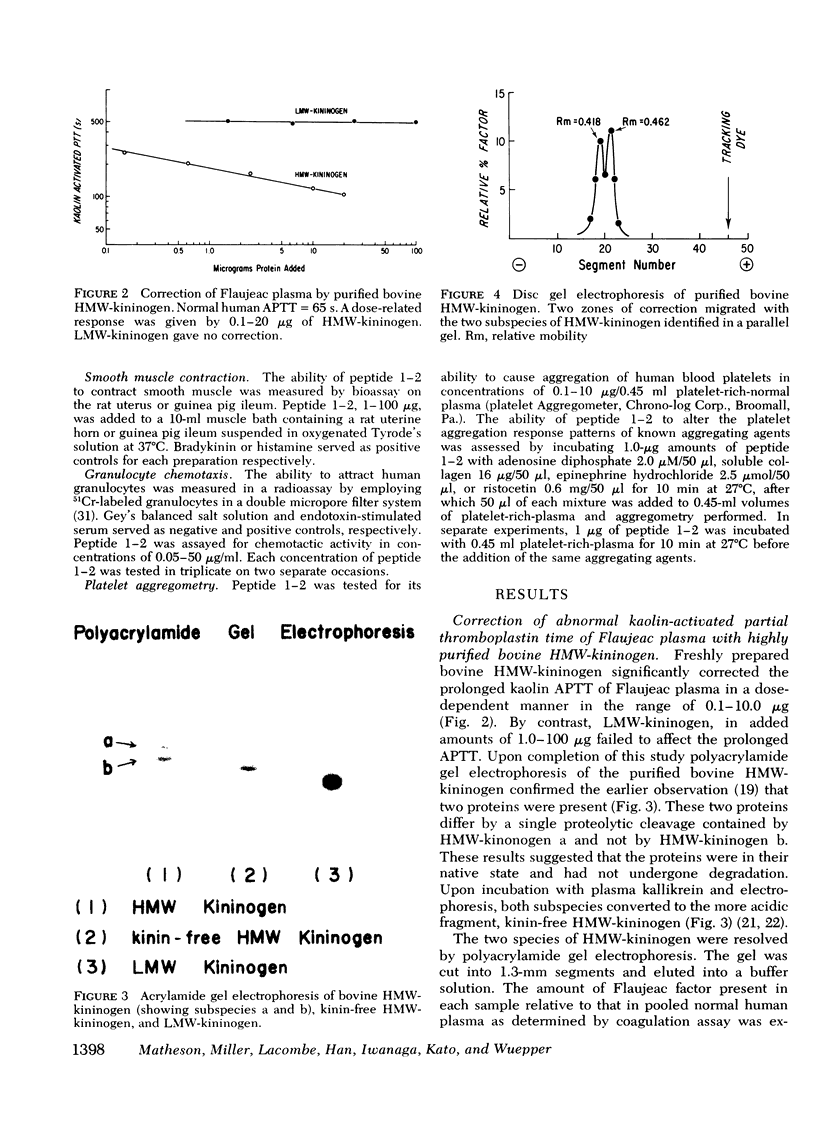
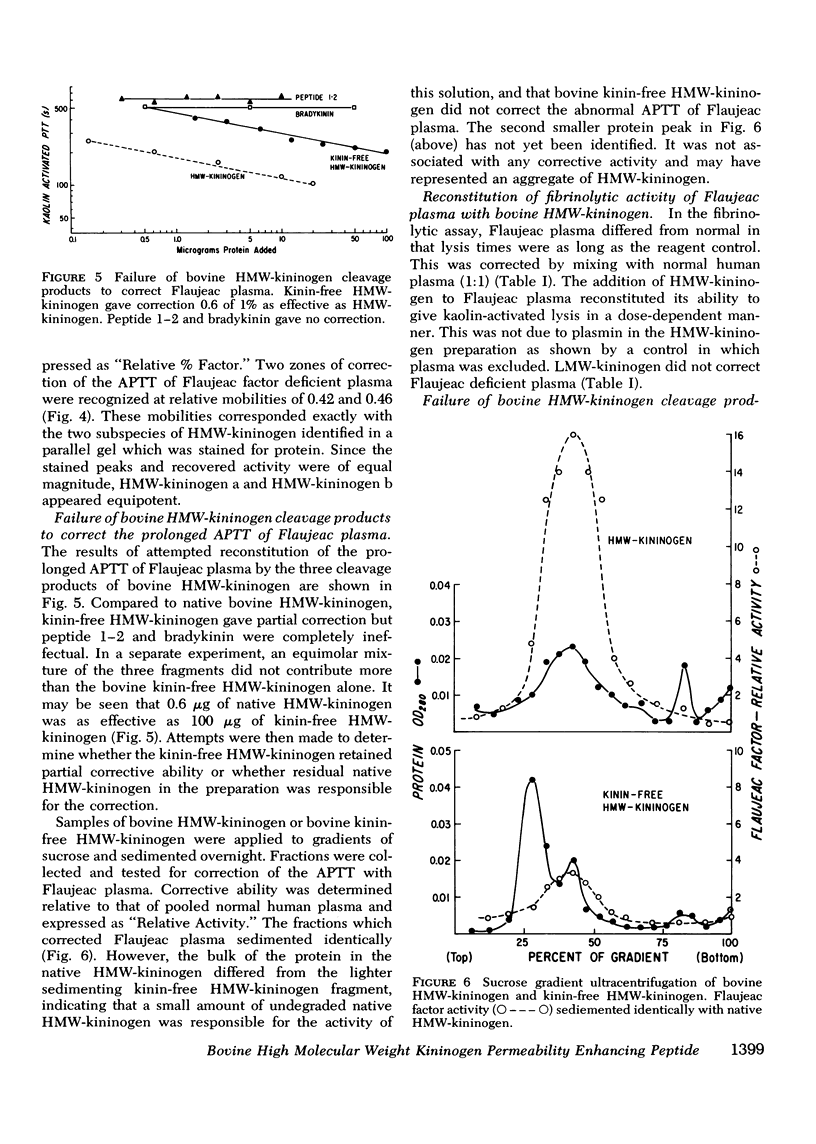
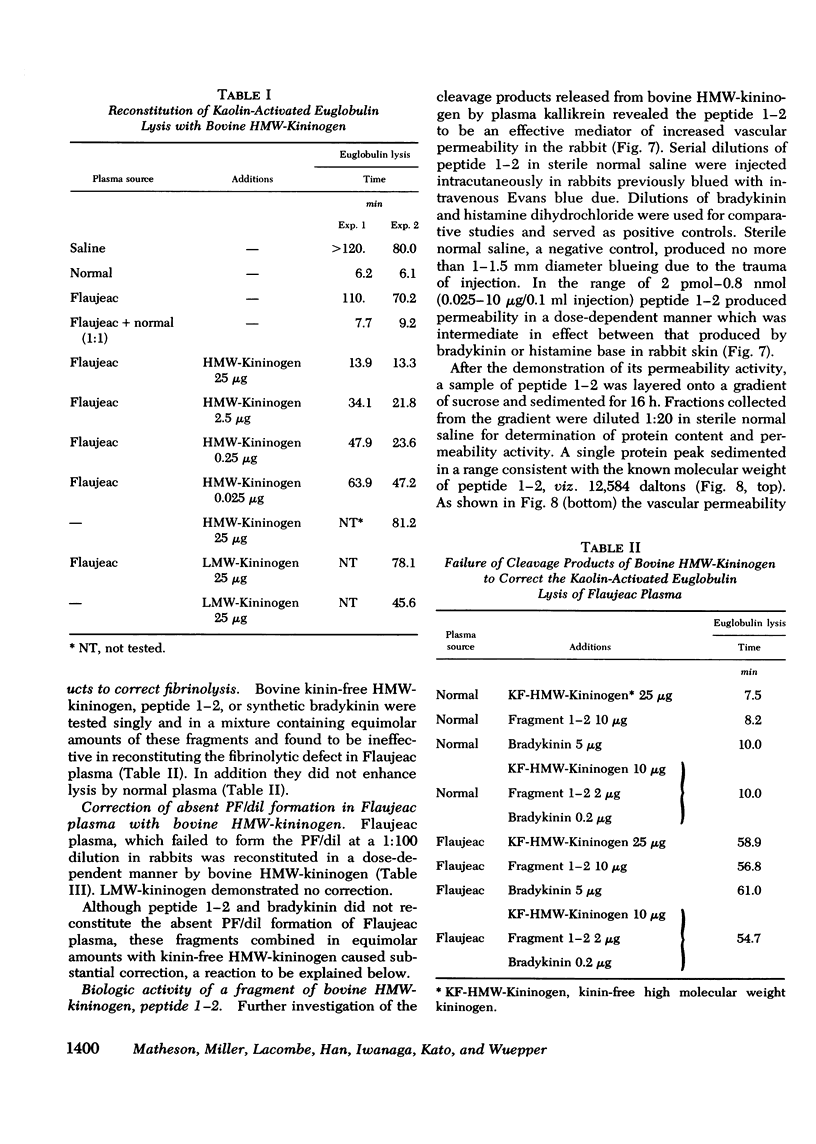
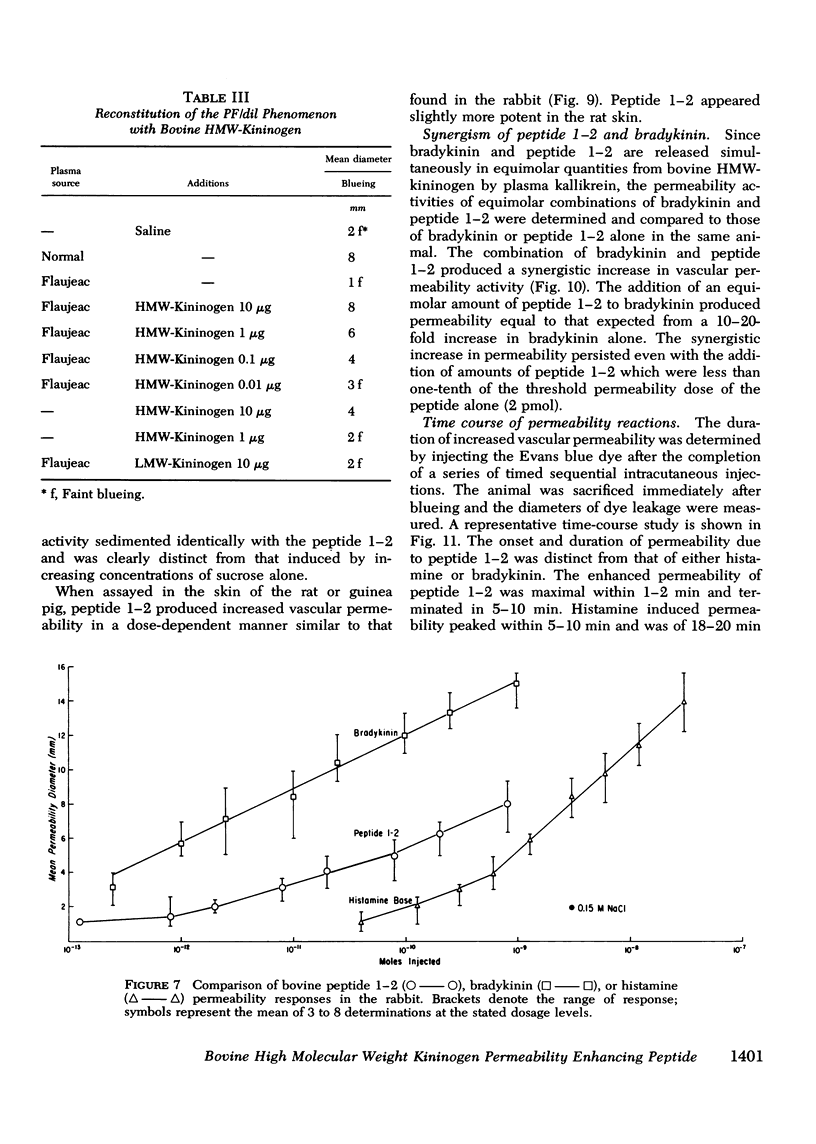
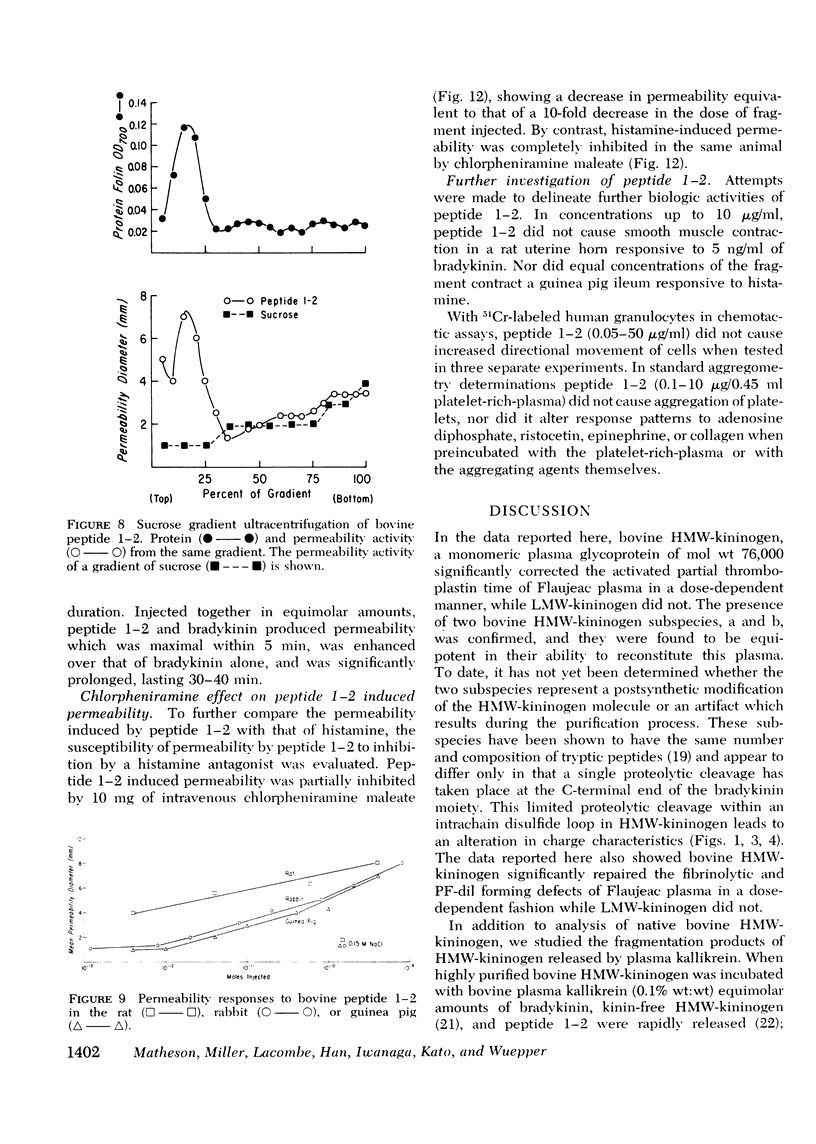
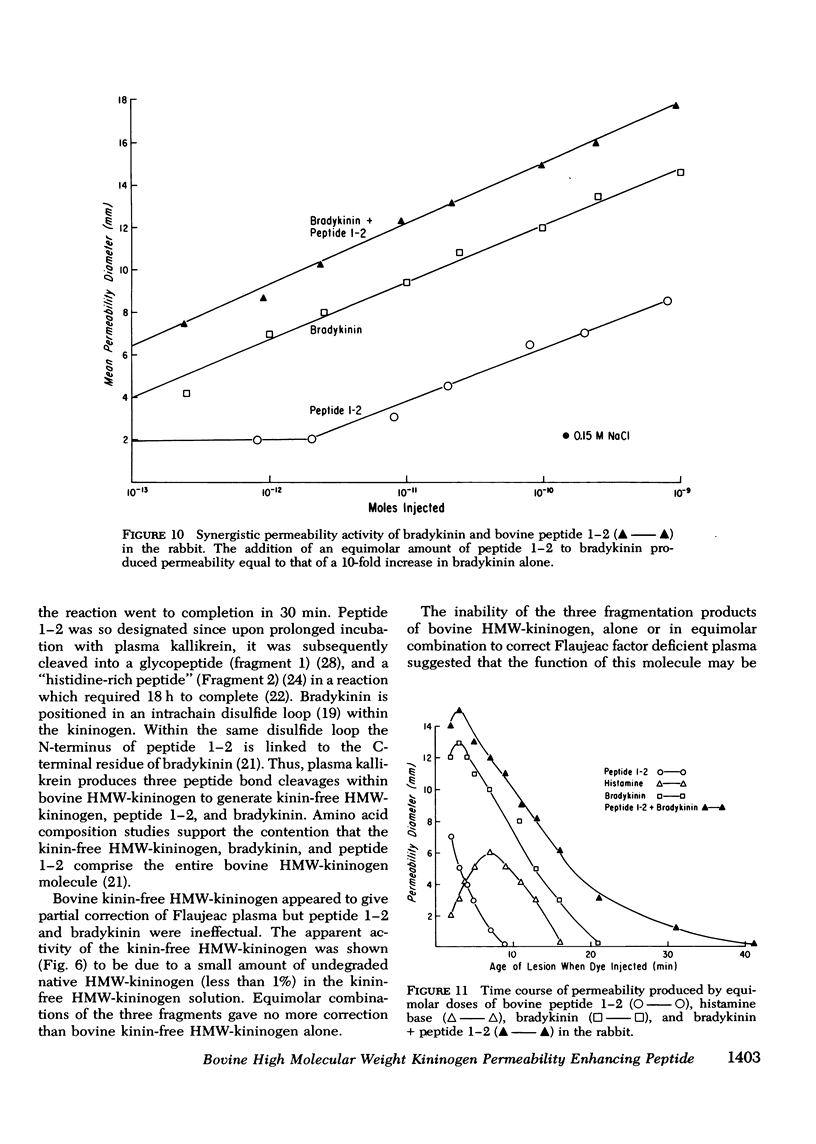
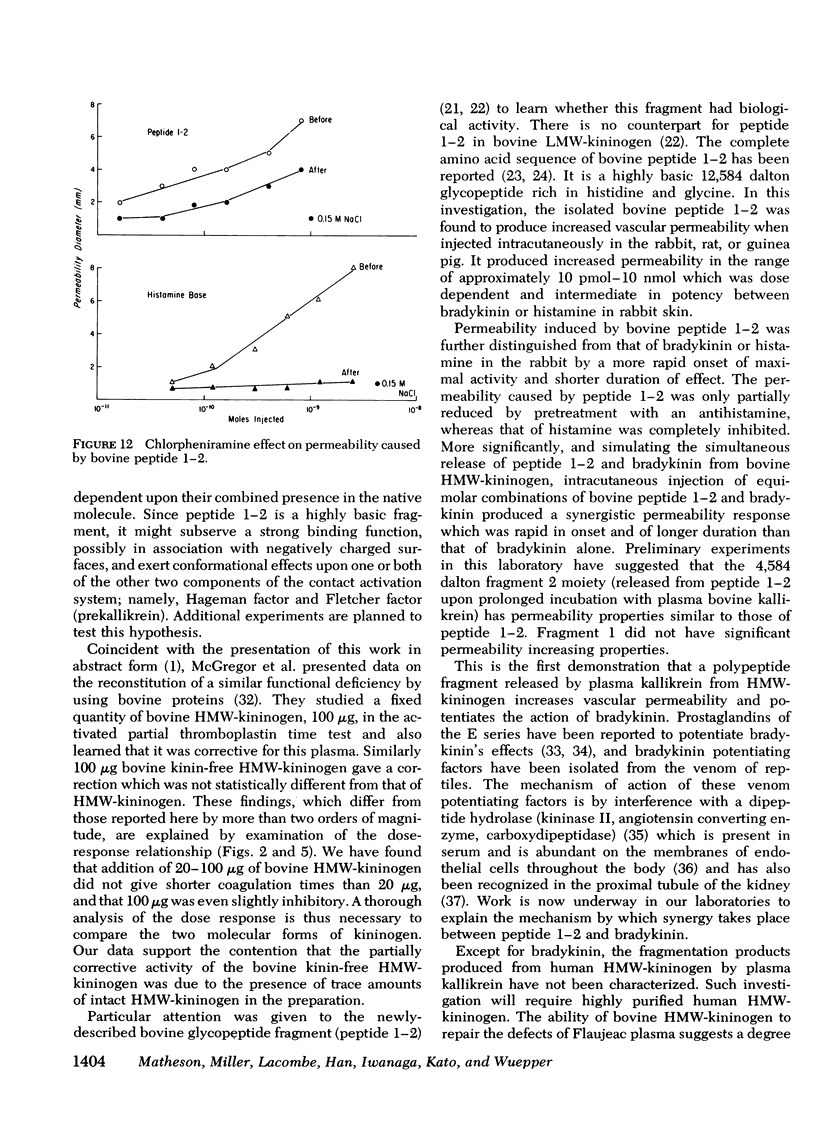
Flaujeac trait is the functional deficiency of a plasma protein of the intrinsic coagulation, kinin-forming, and plasma fibrinolytic pathways. The Flaujeac factor in man has been isolated and tentatively identified as a kininogen of high molecular weight (HMW). Highly purified bovine HMW-kininogen, but not bovine low molecular weight kininogen, repaired Flaujeac factor deficiency. The two subspecies of this molecule, HMW-kininogen a and HMW-kininogen b, also corrected Flaujeac factor deficiency. When bovine HMW-kininogen was incubated with bovine plasma kallikrein, kinin-free HMW-kininogen, bradykinin, and a glycopeptide fragment (peptide 1-2; 12,584 daltons) were rapidly released. None of these fragmentation products corrected Flaujeac factor deficiency alone or in mixtures. The function of HMW-kininogen appeared to depend upon the structural integrity of the native molecule. When injected in concentrations of 2 pmol-8 nmol/0.1 ml, peptide 1-2 caused increased vascular permeability in rabbits, rats, or guinea pigs. The enhanced permeability was maximal within 1-2 min and terminated in 5-10 min, differing from that of bradykinin or histamine. Injected together in equimolar amounts, peptide 1-2 and bradykinin produced a synergistic permeability response which was immediate in onset as well as prolonged in duration. Peptide 1-2 is a rapidly acting, highly basic glyco-peptide which mediates increased vascular permeability in a complementary and synergistic manner with bradykinin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell P. R., Seegal B. C., Hsu K. C., Das M., Soffer R. L. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976 Mar 12;191(4231):1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Colman R. W., Bagdasarian A., Talamo R. C., Scott C. F., Seavey M., Guimaraes J. A., Pierce J. V., Kaplan A. P. Williams trait. Human kininogen deficiency with diminished levels of plasminogen proactivator and prekallikrein associated with abnormalities of the Hageman factor-dependent pathways. J Clin Invest. 1975 Dec;56(6):1650–1662. doi: 10.1172/JCI108247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Donaldson V. H., Glueck H. I., Miller M. A., Movat H. Z., Habal F. Kininogen deficiency in Fitzgerald trait: role of high molecular weight kininogen in clotting and fibrinolysis. J Lab Clin Med. 1976 Feb;87(2):327–337. [PubMed] [Google Scholar]
- Ferreira S. H., Greene L. H., Alabaster V. A., Bakhle Y. S., Vane J. R. Activity of various fractions of bradykinin potentiating factor against angiotensin I converting enzyme. Nature. 1970 Jan 24;225(5230):379–380. doi: 10.1038/225379a0. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Kimball H. R. Granulocyte chemotaxis: an improved in vitro assay employing 51 Cr-labeled granulocytes. J Immunol. 1973 Jan;110(1):233–240. [PubMed] [Google Scholar]
- Han Y. N., Kato H., Iwanaga S., Suzuki T. Bovine plasma high molecular weight kininogen: the amino acid sequence of fragment 1 (glycopeptide) released by the action of plasma kallikrein and its location in the precursor protein. FEBS Lett. 1976 Mar 15;63(1):197–200. doi: 10.1016/0014-5793(76)80225-6. [DOI] [PubMed] [Google Scholar]
- Han Y. N., Komiya M., Iwanaga S., Suzuki T. Studies on the primary structure of bovine high-molecular-weight kininogen. Amino acid sequence of a fragment ("histidine-rich peptide") released by plasma kallikrein. J Biochem. 1975 Jan 1;77(1?):55–68. [PubMed] [Google Scholar]
- Han Y. N., Komiya M., Kato H., Iwanaga S., Suzuki T. Primary structure of bovine high molecular weight kininogen: chemical compositions of kinin-free kininogen and peptide fragments released by plasma kallikrein. FEBS Lett. 1975 Oct 1;57(3):254–258. doi: 10.1016/0014-5793(75)80311-5. [DOI] [PubMed] [Google Scholar]
- Komiya M., Kato H., Suzuki T. Bovine plasma kininogens. I. Further purification of high molecular weight kininogen and its physicochemical properties. J Biochem. 1974 Oct;76(4):811–822. [PubMed] [Google Scholar]
- Komiya M., Kato H., Suzuki T. Bovine plasma kininogens. II. Microheterogeneities of high molecular weight kininogens and their structural relationships. J Biochem. 1974 Oct;76(4):823–832. [PubMed] [Google Scholar]
- Komiya M., Kato H., Suzuki T. Bovine plasma kininogens. III. Structural comparison of high molecular weight and low molecular weight kininogens. J Biochem. 1974 Oct;76(4):833–845. [PubMed] [Google Scholar]
- Komiya M., Nagasawa S., Suzuki T. Bovine prekallikrein activator with functional activity as Hageman factor. J Biochem. 1972 Nov;72(5):1205–1218. doi: 10.1093/oxfordjournals.jbchem.a130008. [DOI] [PubMed] [Google Scholar]
- Lacombe M. J. Déficit constitutionnel en un nouveau facteur de la coagulation intervenant au niveau de contact: le facteur "Flaujeac". C R Acad Sci Hebd Seances Acad Sci D. 1975 Feb 24;280(8):1039–1041. [PubMed] [Google Scholar]
- Lacombe M. J., Varet B., Levy J. P. A hitherto undescribed plasma factor acting at the contact phase of blood coagulation (Flaujeac factor): case report and coagulation studies. Blood. 1975 Nov;46(5):761–768. [PubMed] [Google Scholar]
- MILES A. A., WILHELM D. L. Enzyme-like globulins from serum reproducing the vascular phenomena of inflammation. I. An activable permeability factor and its inhibitor in guinea-pig serum. Br J Exp Pathol. 1955 Feb;36(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Ferreira S. H., Vane J. R. Prostaglandins, aspirin-like drugs and the oedema of inflammation. Nature. 1973 Nov 23;246(5430):217–219. doi: 10.1038/246217a0. [DOI] [PubMed] [Google Scholar]
- Ryan J. W., Ryan U. S., Schultz D. R., Whitaker C., Chung A. Subcellular localization of pulmonary antiotensin-converting enzyme (kininase II). Biochem J. 1975 Feb;146(2):497–499. doi: 10.1042/bj1460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Ratnoff O. D., Waldmann R., Abraham J. P. Fitzgerald Trait: Deficiency of a Hitherto Unrecognized Agent, Fitzgerald Factor, Participating in Surface-Mediated Reactions of Clotting, Fibrinolysis, Generation of Kinins, and the Property of Diluted Plasma Enhancing Vascular Permeability (PF/Dil). J Clin Invest. 1975 May;55(5):1082–1089. doi: 10.1172/JCI108009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Nagasawa S., Suzuki T. Studies on prekallikrein of bovine plasma. I. Purification and properties. J Biochem. 1972 Mar;71(3):471–483. [PubMed] [Google Scholar]
- Williams T. J., Morley J. Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature. 1973 Nov 23;246(5430):215–217. doi: 10.1038/246215a0. [DOI] [PubMed] [Google Scholar]
- Wuepper K. D., Miller D. R., Lacombe M. J. Flaujeac trait. Deficiency of human plasma kininogen. J Clin Invest. 1975 Dec;56(6):1663–1672. doi: 10.1172/JCI108248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuepper K. D. Prekallikrein deficiency in man. J Exp Med. 1973 Dec 1;138(6):1345–1355. doi: 10.1084/jem.138.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M., Nagasawa S., Horiuchi K., Suzuki T. Separation of a new substrate, kininogen-I, for plasma kallikrein in bovine plasma. J Biochem. 1967 Oct;62(4):504–506. doi: 10.1093/oxfordjournals.jbchem.a128698. [DOI] [PubMed] [Google Scholar]
- Yano M., Nagasawa S., Suzuki T. Partial purification and some properties of high molecular weight kininogen, bovine kininogen-I. J Biochem. 1971 Mar;69(3):471–481. [PubMed] [Google Scholar]