Abstract
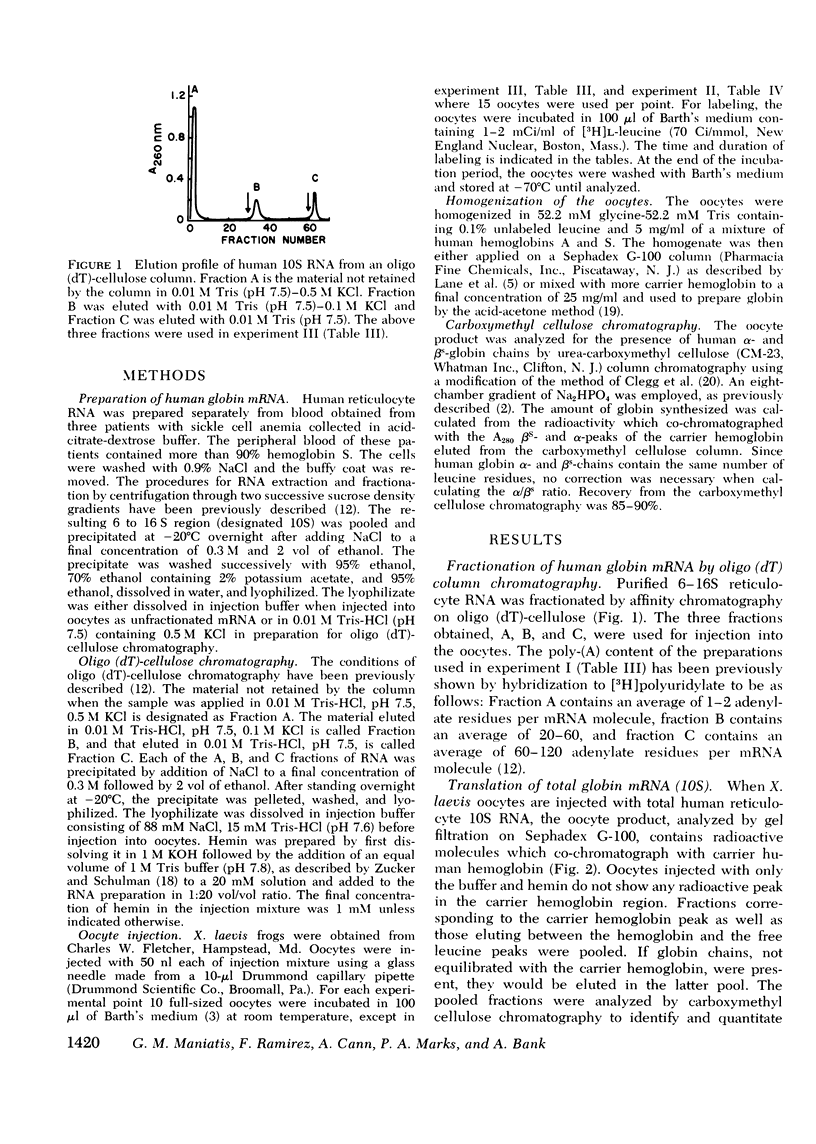
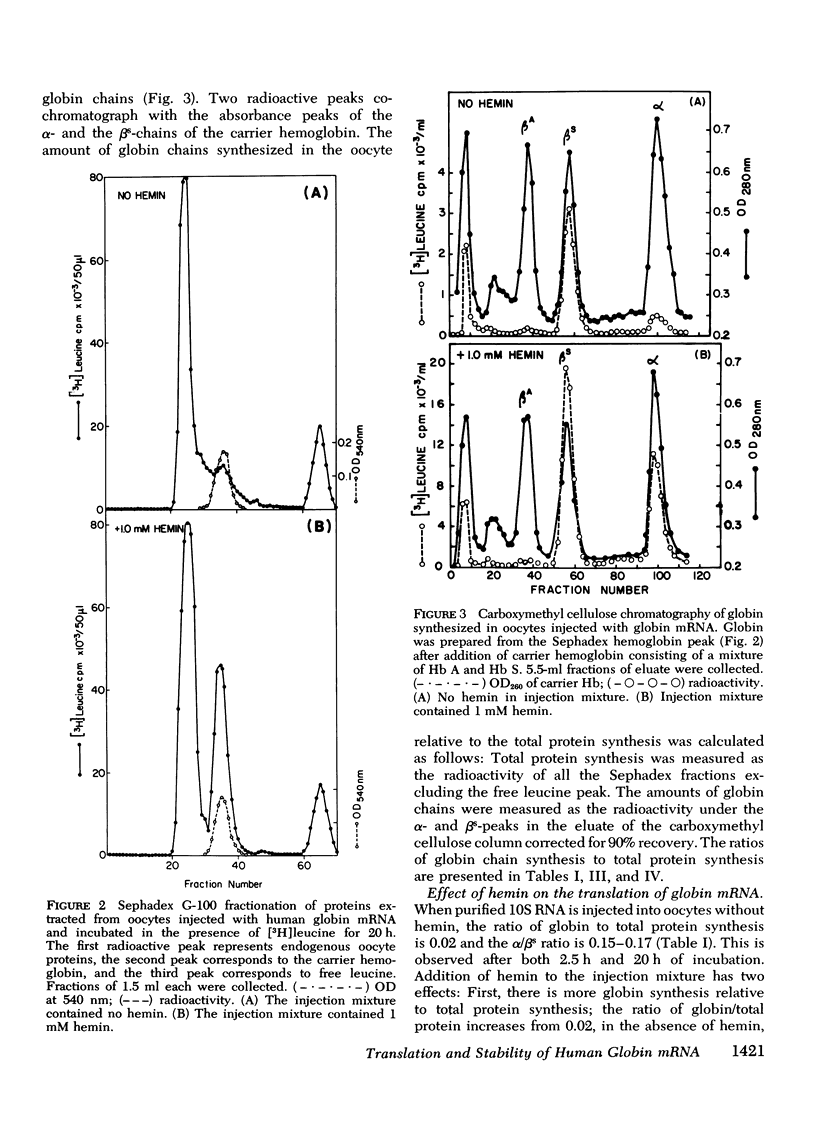
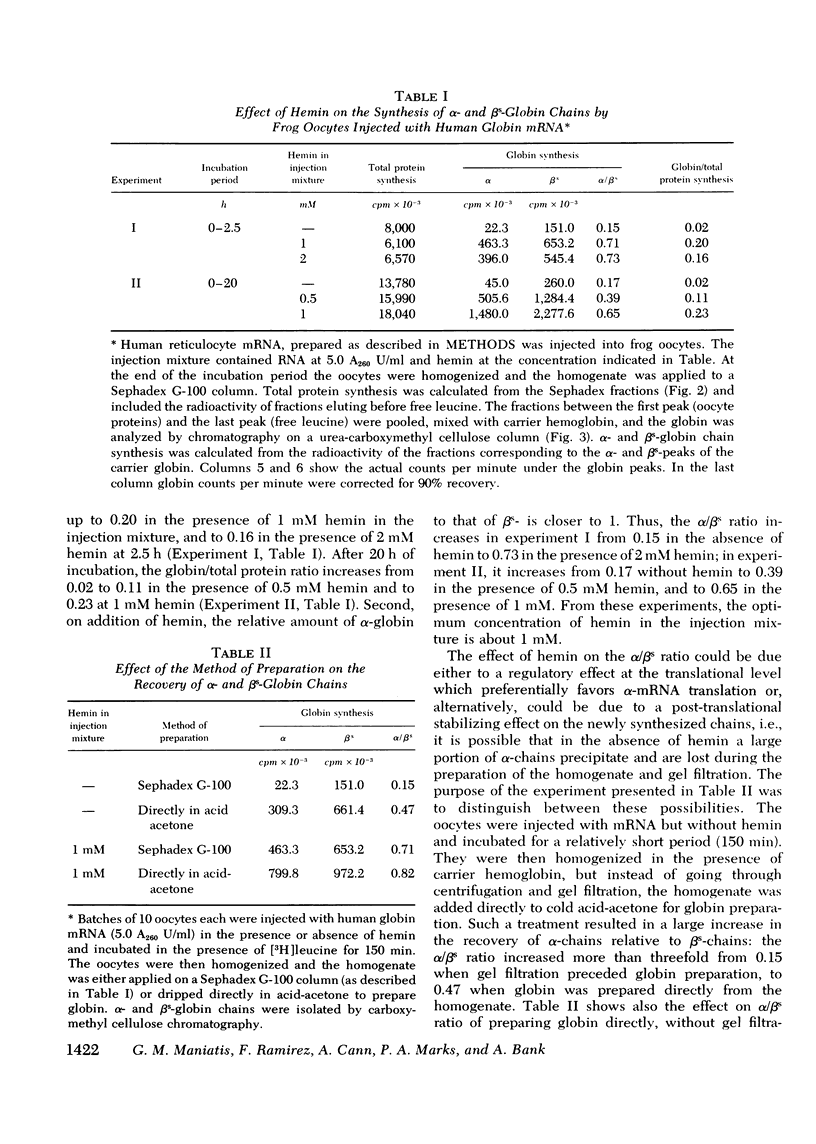
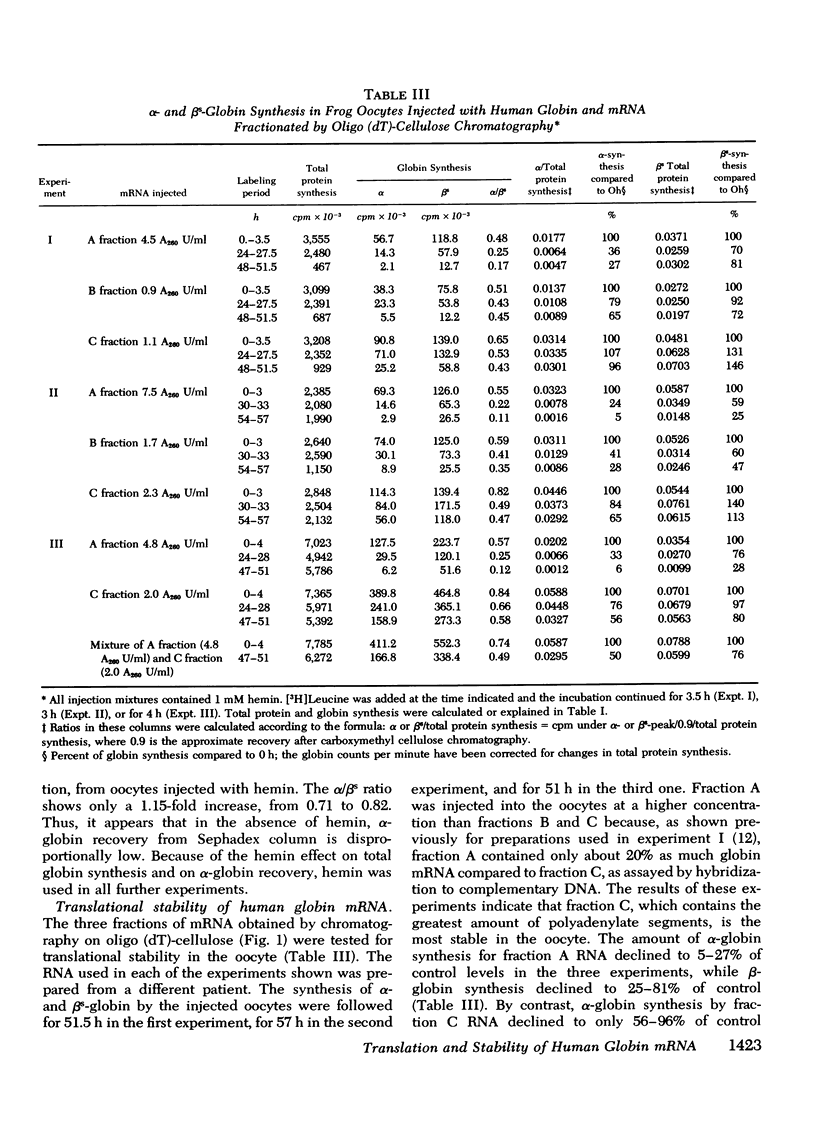
Human globin messenger RNA (mRNA) prepared from erythroid cells of patients with sickle cell anemia has been translated in Xenopus laevis oocytes. Addition of hemin to the injected mRNA causes total globin synthesis to increase and the ratio of alpha- to betas-globin synthesis (alpha/betas ratio) to approach unity. To determine the effect of the length of the poly-(A) segment on human globin mRNA stability, 10 S globin mRNA was fractionated into poly-(A)-poor fractions by oligo (dT)-cellulose column chromatography. When oocytes are injected with each of these fractions, translation of the poly-(A)-rich globin mRNA is sustained for a longer period than that of the poly-(A)-poor mRNA. Regardless of the mRNA fraction injected, the alpha/betas ratio of the synthesized globin decreases as the injected oocytes are incubated for longer periods. The results indicate that in frog oocytes poly-(A)-rich mRNA has greater translational stability than poly-(A)-poor mRNA, AND beta-mRNA has greater stability than alpha-mRNA with comparable poly-(A) content.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Yau P. M., Herbert E., Zucker W. V. Involvement of hemin, a stimulatory fraction from ribosomes and a protein synthesis inhibitor in the regulation of hemoglobin synthesis. J Mol Biol. 1972 Jan 28;63(2):247–264. doi: 10.1016/0022-2836(72)90373-7. [DOI] [PubMed] [Google Scholar]
- Allende C. C., Allende J. E., Firtel R. A. The degradation of ribonucleic acids injected into Xenopus laevis oocytes. Cell. 1974 Jul;2(3):189–196. doi: 10.1016/0092-8674(74)90093-2. [DOI] [PubMed] [Google Scholar]
- Bank A., Gambino R., Ramirez F., Maniatis G., Natta C., Kacian D., Spiegelman S., Marks P. A. Regulation of globin synthesis in the thalassemias. Ann N Y Acad Sci. 1974 Nov 29;241(0):247–252. doi: 10.1111/j.1749-6632.1974.tb21883.x. [DOI] [PubMed] [Google Scholar]
- Beuzard Y., London I. M. The effects of hemin and double-stranded RNA on alpha and beta globin synthesis in reticulocyte and Krebs II ascites cell-free systems and the relationship of these effects to an initiation factor preparation. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2863–2866. doi: 10.1073/pnas.71.7.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzard Y., Rodvien R., London I. M. Effect of hemin on the synthesis of hemoglobin and other proteins in mammalian cells. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1022–1026. doi: 10.1073/pnas.70.4.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann A., Gambino R., Banks J., Bank A. Polyadenylate sequences and biologic activity of human globin messenger ribonucleic acid. J Biol Chem. 1974 Dec 10;249(23):7536–7540. [PubMed] [Google Scholar]
- Chan L., Kohler P. O., O'Malley B. W. Translation of ovalbumin mRNA in Xenopus laevis oocytes. Characterization of the system and effects of estrogen on injected mRNA populations. J Clin Invest. 1976 Mar;57(3):576–585. doi: 10.1172/JCI108313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Giglioni B., Gianni A. M., Comi P., Ottolenghi S., Rungger D. Translational control of globin synthesis by haemin in Xenopus oocytes. Nat New Biol. 1973 Nov 28;246(152):99–102. doi: 10.1038/newbio246099a0. [DOI] [PubMed] [Google Scholar]
- Gorski J., Morrison M. R., Merkel C. G., Lingrel J. B. Size heterogeneity of polyadenylate sequences in mouse globin messenger RNA. J Mol Biol. 1974 Jun 25;86(2):363–371. doi: 10.1016/0022-2836(74)90025-4. [DOI] [PubMed] [Google Scholar]
- Grayzel A. I., Hörchner P., London I. M. The stimulation of globin synthesis by heme. Proc Natl Acad Sci U S A. 1966 Mar;55(3):650–655. doi: 10.1073/pnas.55.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lingrel J. B., Marbaix G. Message stability in injected frog oocytes: long life of mammalian alpha and beta globin messages. J Mol Biol. 1973 Nov 5;80(3):539–551. doi: 10.1016/0022-2836(73)90421-x. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., LeGendre S. M., Levy C. C. Stabilization of an RNA molecule by 3'-terminal poly (A)-induced inhibition of RNase activity. J Biol Chem. 1976 Jun 10;251(11):3287–3293. [PubMed] [Google Scholar]
- Housman D., Forget B. G., Skoultchi A., Benz E. J., Jr Quantitative deficiency of chain-specific globin messenger ribonucleic acids in the thalassemia syndromes. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1809–1813. doi: 10.1073/pnas.70.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Marbaix G., Hubert E., Leclercq M., Nudel U., Soreq H., Salomon R., Lebleu B., Revel M., Littauer U. Z. Role of the polyadenylate segment in the translation of globin messenger RNA in Xenopus oocytes. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3143–3146. doi: 10.1073/pnas.71.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C. D., Gurdon J. B., Woodland H. R. Control of translation of globin mRNA in embryonic cells. Nature. 1974 Oct 4;251(5474):436–437. doi: 10.1038/251436a0. [DOI] [PubMed] [Google Scholar]
- Lane C. D., Marbaix G., Gurdon J. B. Rabbit haemoglobin synthesis in frog cells: the translation of reticulocyte 9 s RNA in frog oocytes. J Mol Biol. 1971 Oct 14;61(1):73–91. doi: 10.1016/0022-2836(71)90207-5. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Mathews M. B., Hunt T., Brayley A. Specificity of the control of protein synthesis by haemin. Nat New Biol. 1973 Jun 20;243(129):230–233. doi: 10.1038/newbio243230a0. [DOI] [PubMed] [Google Scholar]
- Maxwell C. R., Kamper C. S., Rabinovitz M. Hemin control of globin synthesis: an assay for the inhibitor formed in the absence of hemin and some characteristics of its formation. J Mol Biol. 1971 May 28;58(1):317–327. doi: 10.1016/0022-2836(71)90249-x. [DOI] [PubMed] [Google Scholar]
- Metafora S., Terada M., Dow L. W., Marks P. A., Bank A. Increased efficiency of exogenous messenger RNA translation in a Krebs ascites cell lysate. Proc Natl Acad Sci U S A. 1972 May;69(5):1299–1303. doi: 10.1073/pnas.69.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Anderson W. F. Isolation and translation of hemoglobin messenger RNA from thalassemia, sickle cell anemia, and normal human reticulocytes. J Clin Invest. 1971 Nov;50(11):2458–2460. doi: 10.1172/JCI106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudel U., Soreq H., Littauer U. Z. Globin mRNA species containing poly(A) segments of different lengths. Their functional stability in Xenopus oocytes. Eur J Biochem. 1976 Apr 15;64(1):115–121. doi: 10.1111/j.1432-1033.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Puckett L., Darnell J. E. Possible relationship of poly(A) shortening to mRNA turnover. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1077–1081. doi: 10.1073/pnas.72.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A. E., Stavrianopoulos J. G., Schutz G., Feigelson P. Translational properties of rabbit globin mRNA after specific removal of poly(A) with ribonuclease H. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4635–4639. doi: 10.1073/pnas.71.11.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Nudel U., Salomon R., Revel M., Littauer U. Z. In vitro translation of polyadenylic acid-free rabbit globin messenger RNA. J Mol Biol. 1974 Sep 5;88(1):233–245. doi: 10.1016/0022-2836(74)90307-6. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Specific IgG mRNA molecules from myeloma cells in heterogeneous nuclear and cytoplasmic RNA containing poly-A. Nature. 1972 Sep 15;239(5368):143–146. doi: 10.1038/239143a0. [DOI] [PubMed] [Google Scholar]
- Williamson R., Crossley J., Humphries S. Translation of mouse globin messenger ribonucleic acid from which the poly(adenylic acid) sequence has been removed. Biochemistry. 1974 Feb 12;13(4):703–707. doi: 10.1021/bi00701a011. [DOI] [PubMed] [Google Scholar]
- Williamson R., Drewienkiewicz C. E., Paul J. Globin messenger sequences in high molecular weight RNA from embryonic mouse liver. Nat New Biol. 1973 Jan 17;241(107):66–68. doi: 10.1038/newbio241066a0. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Hew C. L., Hsu H. Translation of messenger ribonucleic acid from isolated pancreatic islets and human insulinomas. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4777–4779. doi: 10.1073/pnas.72.12.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker W. V., Schulman H. M. The synthesis of globin dimers by a reticulocyte cell-free system. Biochim Biophys Acta. 1967 Apr 18;138(2):400–410. doi: 10.1016/0005-2787(67)90499-6. [DOI] [PubMed] [Google Scholar]



