Abstract
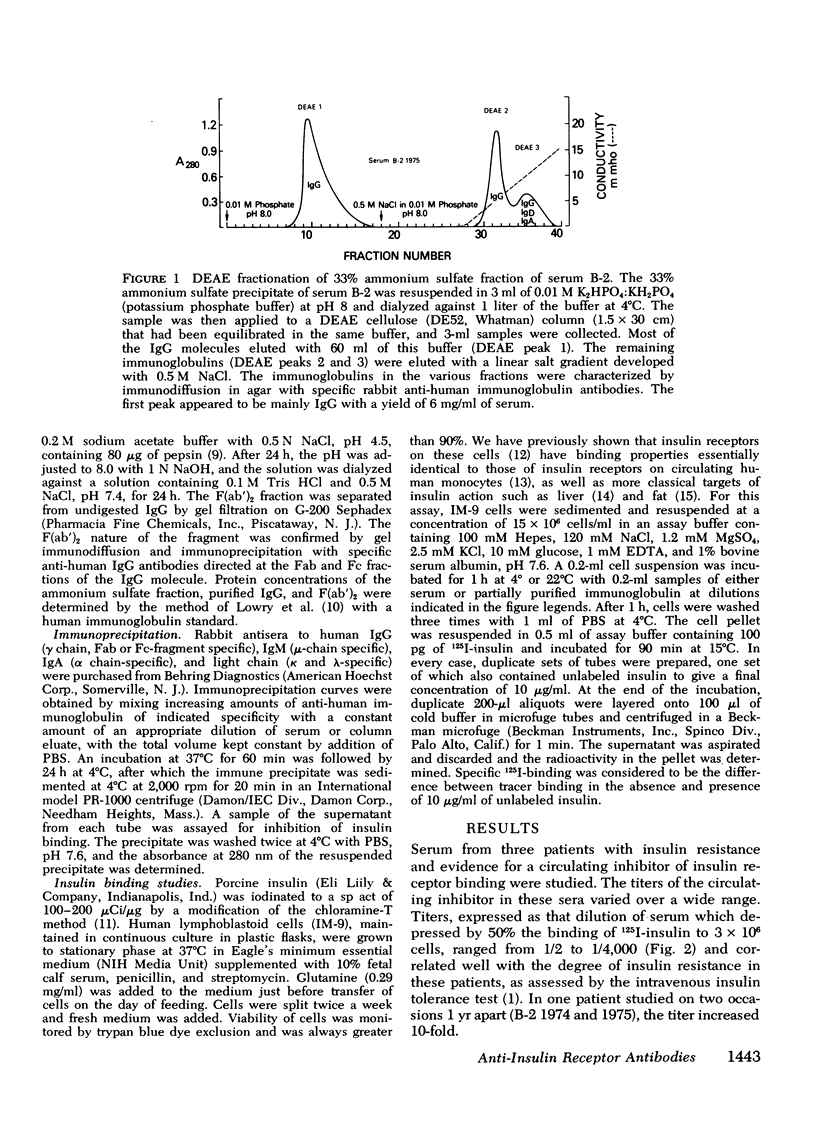
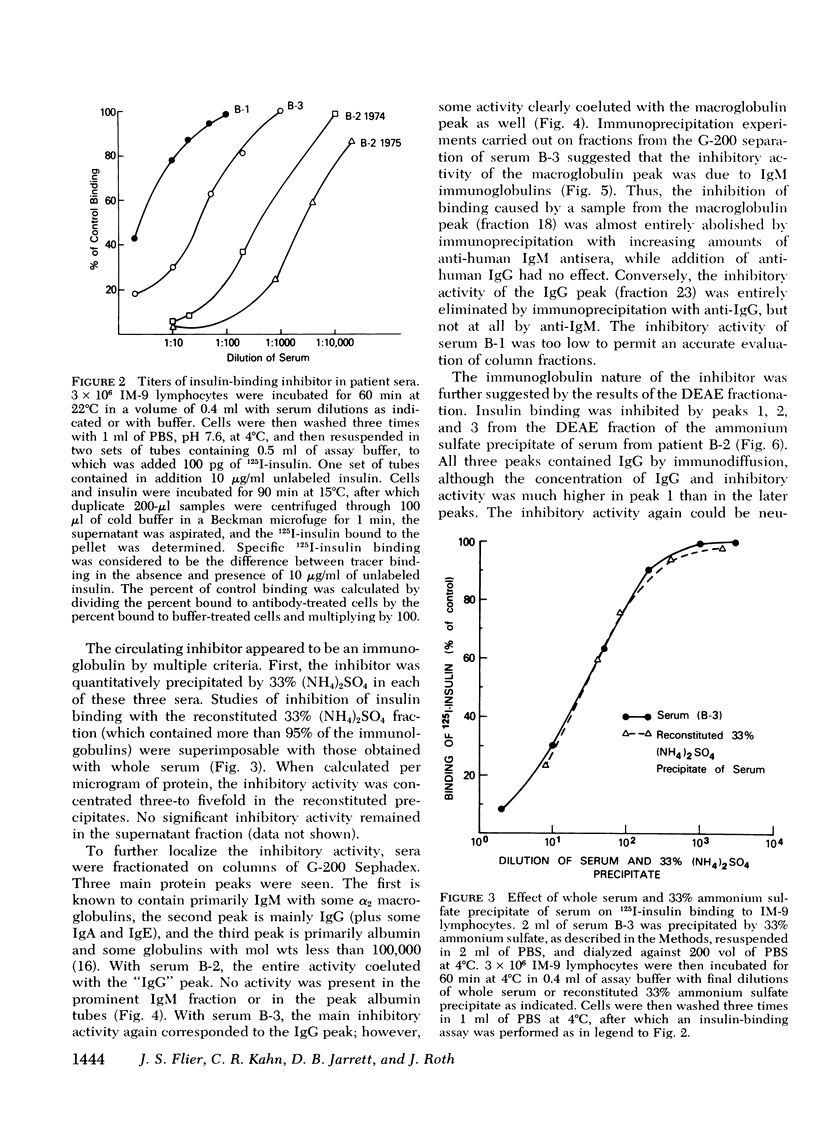
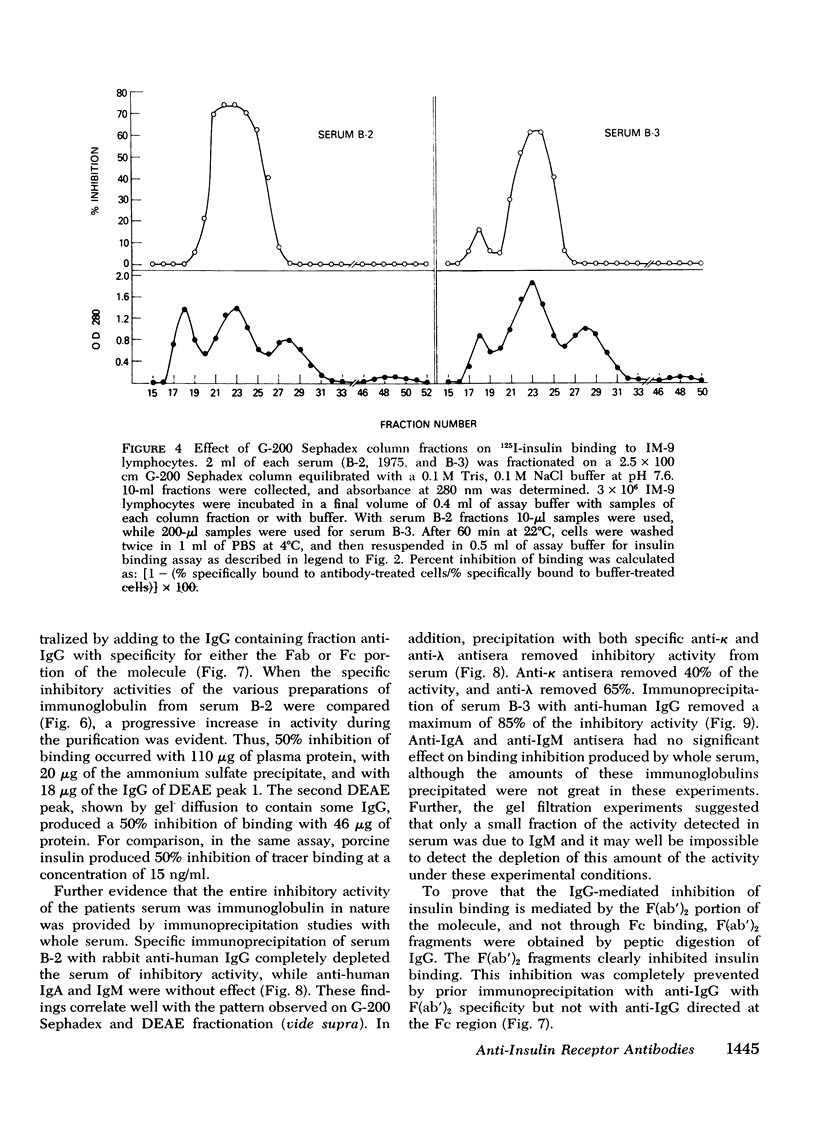
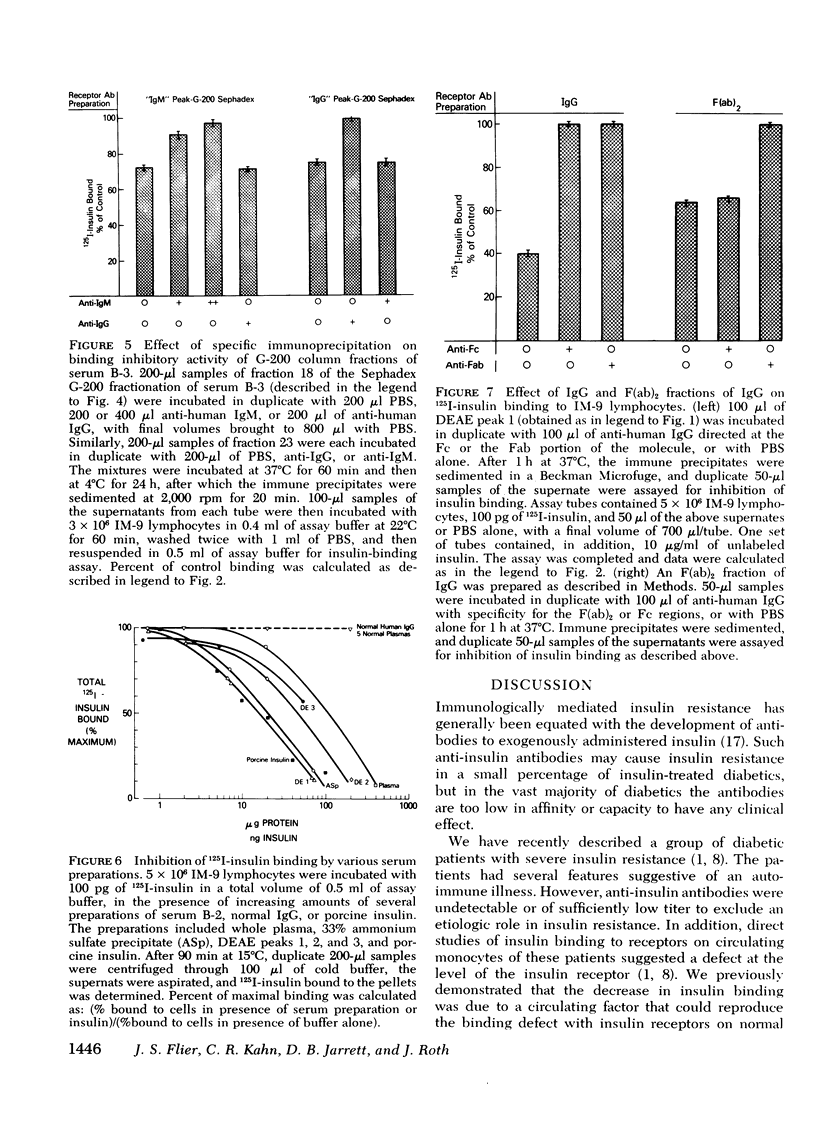
We have characterized the circulating inhibitor of insulin receptor binding found in several patients with a new syndrome of extreme insulin resistance. The inhibitor is an immunoglobulin by multiple criteria, including precipitation by 33% ammonium sulfate, migration on G-200 Sephadex gel filtration and DEAE chromatography, and immuno-precipitation with specific anti-human immuno-globulins. Although predominantly IgG, some activity is found in the IgM fraction of the immunoglobulins in one patient. The inhibitory immunoglobulins reacted with antisera to both kappa and lambda light chain determinants and are therefore polyclonal. In addition, activity is retained in the F(ab')2 fraction of pepsin-digested IgG. Evidence suggests that these antibodies are directed at determinants on or near the insulin receptor, and that they are responsible for the observed clinical insulin resistance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. D., Fastier F. N., Howie J. B., Kennedy T. H., Kilpatrick J. A., Stewart R. D. Stimulation of the human thyroid by infusions of plasma containing LATS protector. J Clin Endocrinol Metab. 1974 Nov;39(5):826–832. doi: 10.1210/jcem-39-5-826. [DOI] [PubMed] [Google Scholar]
- Aharonov A., Abramsky O., Tarrab-Hazdai R., Fuchs S. Humoral antibodies to acetylcholine receptor in patients with myasthenia gravis. Lancet. 1975 Aug 23;2(7930):340–342. doi: 10.1016/s0140-6736(75)92779-8. [DOI] [PubMed] [Google Scholar]
- Almon R. R., Andrew C. G., Appel S. H. Serum globulin in myasthenia gravis: inhibition of alpha-bungarotoxin binding to acetylcholine receptors. Science. 1974 Oct 4;186(4158):55–57. doi: 10.1126/science.186.4158.55. [DOI] [PubMed] [Google Scholar]
- Barnes N. D., Palumbo P. J., Hayles A. B., Folgar H. Insulin resistance, skin changes, and virilization: a recessively inherited syndrome possibly due to pineal gland dysfunction. Diabetologia. 1974 Aug;10(4):285–289. doi: 10.1007/BF02627729. [DOI] [PubMed] [Google Scholar]
- Bender A. N., Ringel S. P., Engel W. K., Daniels M. P., Vogel Z. Myasthenia gravis: a serum factor blocking acetylcholine receptors of the human neuromuscular junction. Lancet. 1975 Mar 15;1(7907):607–609. doi: 10.1016/s0140-6736(75)91886-3. [DOI] [PubMed] [Google Scholar]
- Bruce D. H., Bernard W., Blackard W. G. Spontaneous disappearance of insulin-resistant diabetes mellitus in a patient with a collagen disease. A case report, with review of the literature for conditions associated with insulin resistance. Am J Med. 1970 Feb;48(2):268–272. doi: 10.1016/0002-9343(70)90125-7. [DOI] [PubMed] [Google Scholar]
- FIELD J. B., JOHNSON P., HERRING B. Insulin-resistant diabetes associated with increased endogenous plasma insulin followed by complete remission. J Clin Invest. 1961 Sep;40:1672–1683. doi: 10.1172/JCI104390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Roth J., Bar R. S. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science. 1975 Oct 3;190(4209):63–65. doi: 10.1126/science.170678. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Givens J. R., Kerber I. J., Wiser W. L., Andersen R. N., Coleman S. A., Fish S. A. Remission of acanthosis nigricans associated with polycystic ovarian disease and a stromal luteoma. J Clin Endocrinol Metab. 1974 Mar;38(3):347–355. doi: 10.1210/jcem-38-3-347. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Gammeltoft S. The biological activity and the binding affinity of modified insulins determined on isolated rat fat cells. Diabetologia. 1974 Apr;10(2):105–113. doi: 10.1007/BF01219665. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Flier J. S., Bar R. S., Archer J. A., Gorden P., Martin M. M., Roth J. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976 Apr 1;294(14):739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Kendall-Taylor P. Effects of long-acting thyroid stimulator (LATS) and LATS protector on human thyroid adenyl cyclase activity. Br Med J. 1973 Jul 14;3(5871):72–75. doi: 10.1136/bmj.3.5871.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lennon V. A., Carnegie P. R. Immunopharmacological disease: a break in tolerance to receptor sites. Lancet. 1971 Mar 27;1(7700):630–633. doi: 10.1016/s0140-6736(71)91557-1. [DOI] [PubMed] [Google Scholar]
- Levey G. S., Pastan I. Activation of thyroid adenyl cyclase by long-acting thyroid stimulator. Life Sci I. 1970 Jan 15;9(2):67–73. doi: 10.1016/0024-3205(70)90018-4. [DOI] [PubMed] [Google Scholar]
- McKENZIE J. M. The bioassay of thyrotropin in serum. Endocrinology. 1958 Sep;63(3):372–382. doi: 10.1210/endo-63-3-372. [DOI] [PubMed] [Google Scholar]
- Mukhtar E. D., Smith B. R., Pyle G. A., Hall R., Vice P. Relation of thyroid-stimulating immunoglobulins to thyroid function and effects of surgery, radioiodine, and antithyroid drugs. Lancet. 1975 Mar 29;1(7909):713–715. doi: 10.1016/s0140-6736(75)91629-3. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H., Bianco A. R., Handwerger B. S., Kahn C. R. Demonstration that monocytes rather than lymphocytes are the insulin-binding cells in preparations of humah peripheral blood mononuclear leukocytes: implications for studies of insulin-resistant states in man. Proc Natl Acad Sci U S A. 1975 Feb;72(2):474–478. doi: 10.1073/pnas.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Thyroid-stimulating immunoglobulins in Graves' disease. Lancet. 1974 Aug 24;2(7878):427–431. doi: 10.1016/s0140-6736(74)91815-7. [DOI] [PubMed] [Google Scholar]
- TUCKER W. R., KLINK D., GOETZ F., ZALME E., KNOWLES H. C., Jr INSULIN RESISTANCE AND ACANTHOSIS NIGRICANS. Diabetes. 1964 Jul-Aug;13:395–399. doi: 10.2337/diab.13.4.395. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Field J. B. Effects of long-acting thyroid stimulator on thyrotropin stimulation of adenyl cyclase activity in thyroid plasma membranes. J Clin Invest. 1972 Mar;51(3):463–472. doi: 10.1172/JCI106834. [DOI] [PMC free article] [PubMed] [Google Scholar]



