Abstract
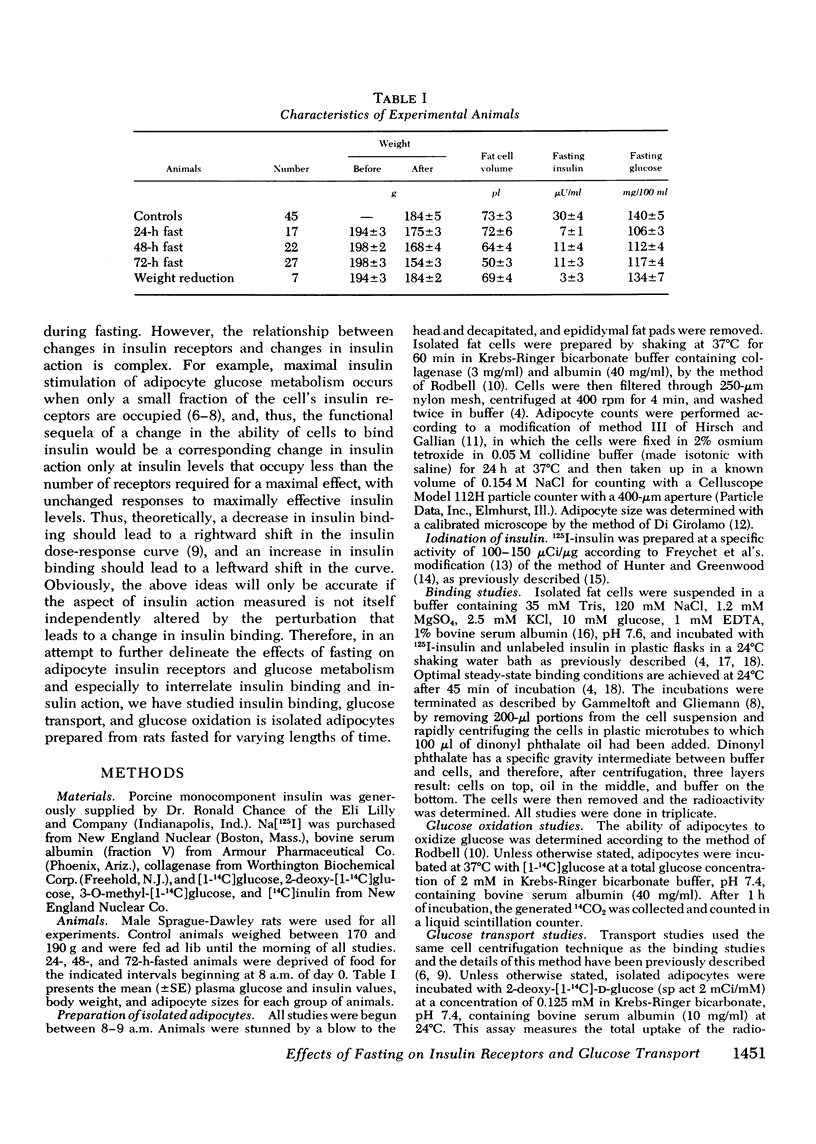
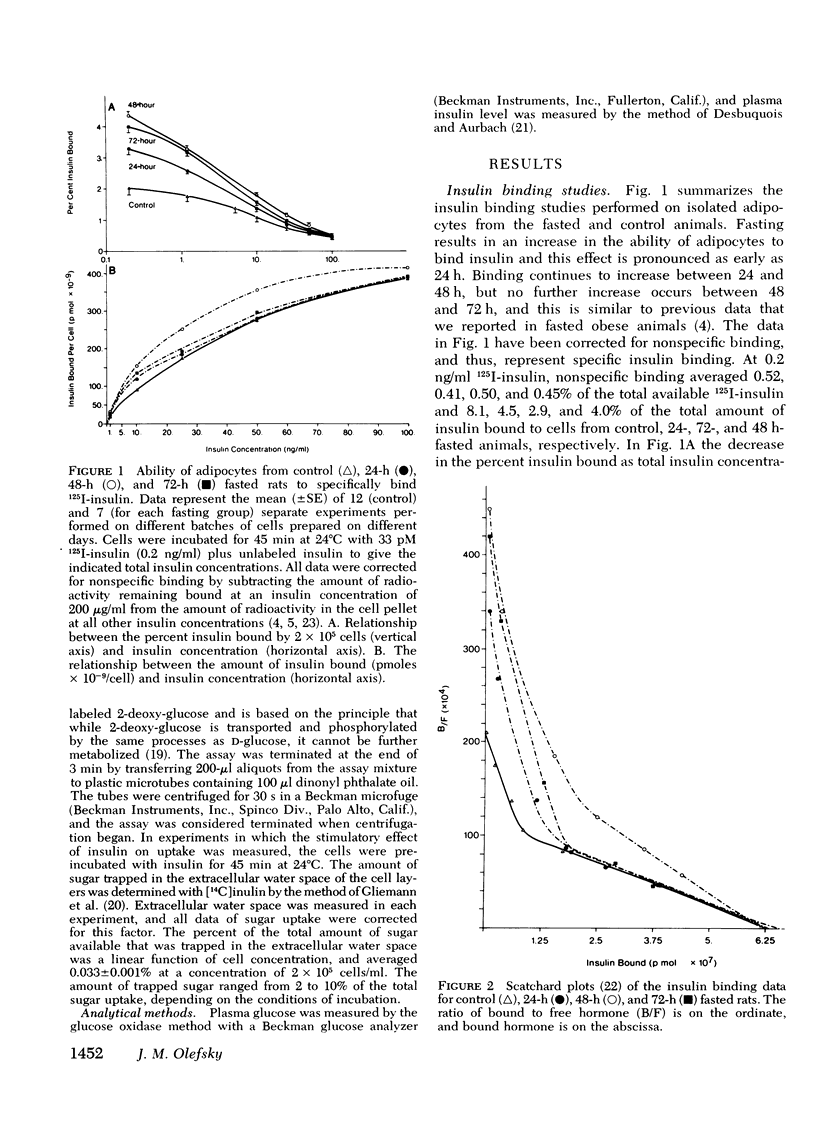
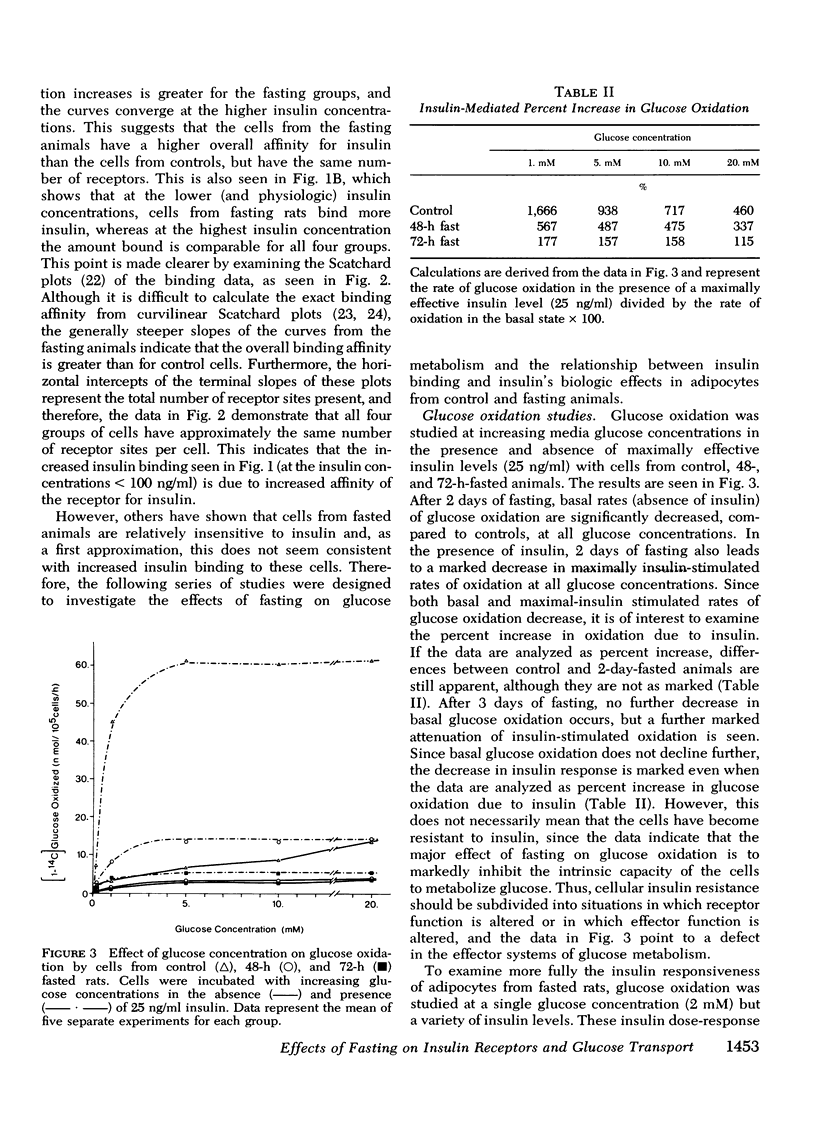
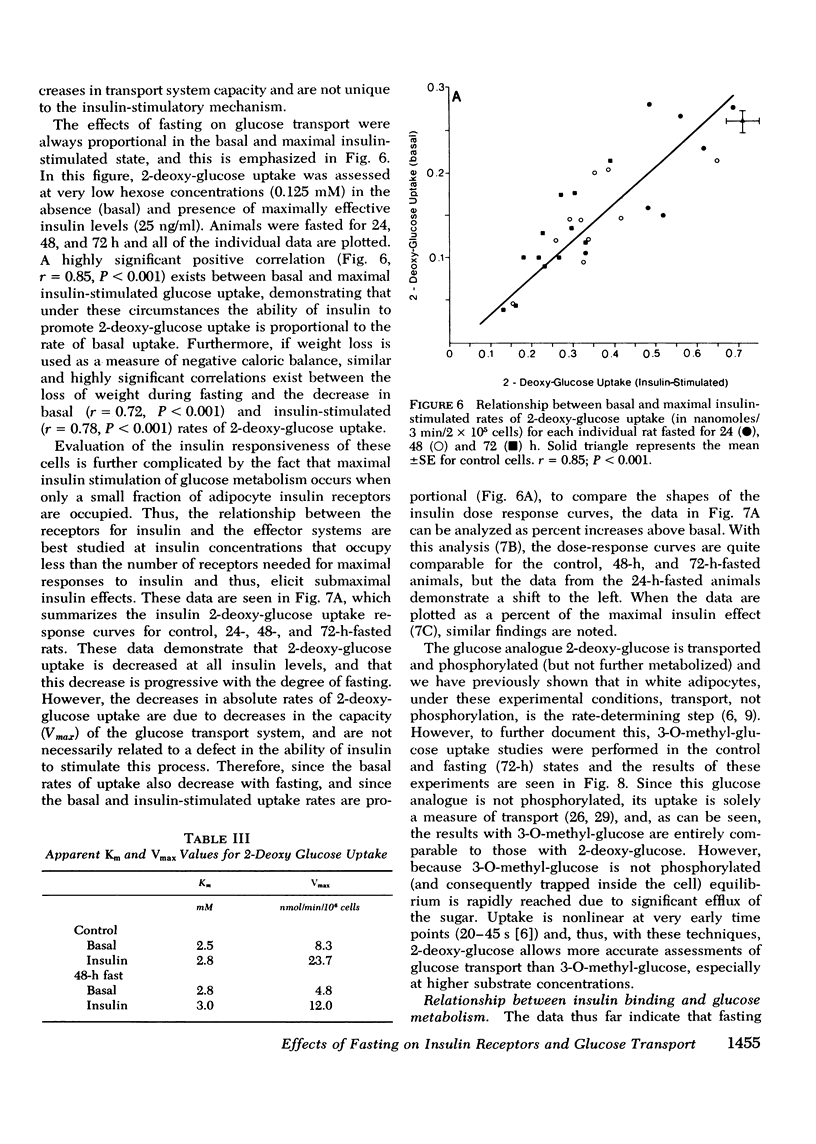
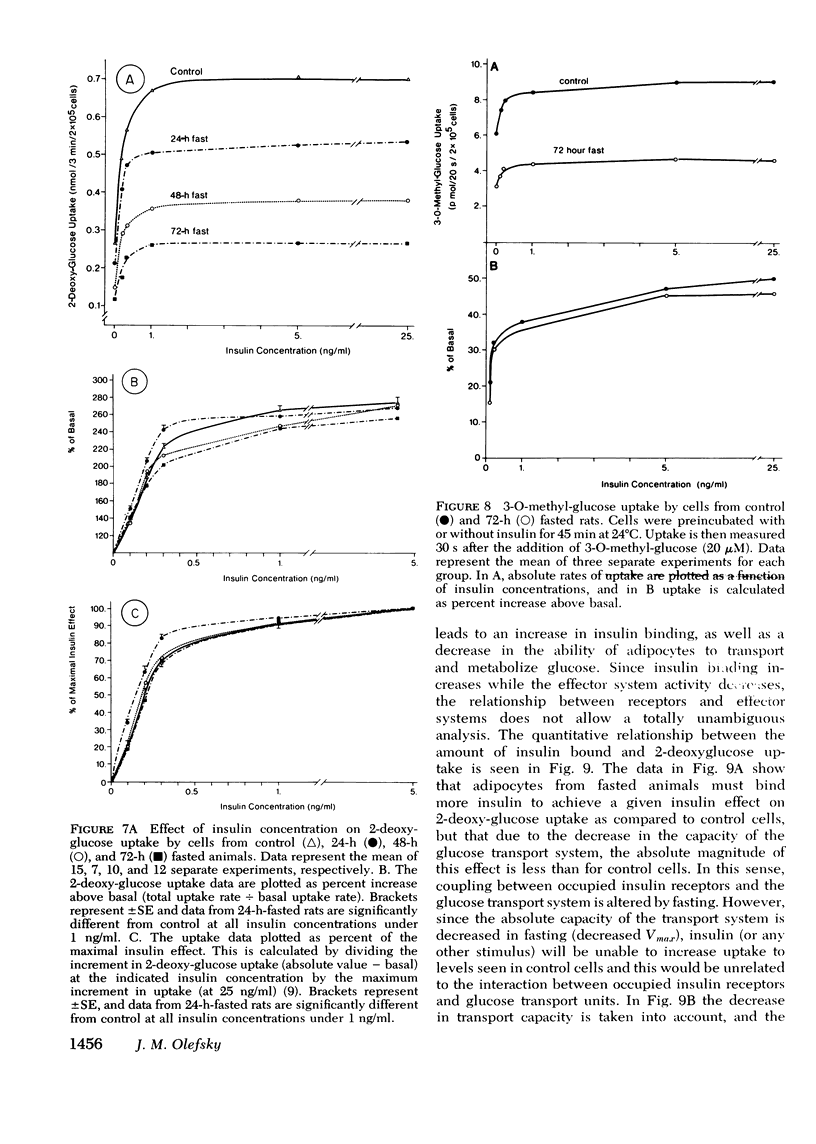
Insulin binding, glucose transport, and glucose oxidation were studied in isolated adipocytes obtained from fasting rats. Fasting led to an increase in the overall binding affinity for insulin, while the number of receptor sites per cell remained constant. Glucose oxidation was markedly attenuated during fasting. Basal rates of oxidation decreased by about 50%, while insulin-stimulated rates decreased 6 to 10-fold. Glucose transport was assessed by measuring initial uptake rate of 2-deoxy-glucose. Fasting led to a 40-50% decrease in the apparent maximal transport capacity (Vmax) of 2-deoxy-glucose uptake with no change in apparent Km. A progressive decrease in basal and insulin-stimulated rates of 2-deoxy-glucose uptake was seen from 24-72 h of starvation and a significant correlation (r=0.85, P less than 0.001) existed between basal and maximal insulin-stimulated uptake rates in individual animals. When 2-deoxy-glucose uptake was plotted as a function of insulin bound, due to the decrease in maximal uptake capacity, cells from fasting animals took up less hexose for any amount of insulin bound. When the insulin bound was plotted as a function of the percent insulin effect on uptake, control cells and cells from 24-h-fasted rats gave comparable results, while cells from 48- and 72-h-fasted animals still took up less hexose for any amount of bound insulin. The effects of fasting on 3-O-methyl glucose uptake were comparable to the 2-deoxy-glucose data. In conclusion: (a) insulin binding is increased during fasting due to an increased overall binding affinity with no change in receptor number; (b) glucose oxidation is severely impaired during fasting; (c) 2-deoxy-glucose uptake decreases with fasting due to a decrease in maximal transport capacity (Vmax) with no change in Km; (d) the decrease in glucose oxidation is much greater than the decrease in glucose transport, indicating impaired intracellular oxidative metabolism; and (e) coupling between insulin receptors and the glucose transport system is normal after 24 h of fasting but is impaired at 48 and 72 h.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Hexose transport in isolated brown fat cells. A model system for investigating insulin action on membrane transport. J Biol Chem. 1974 Sep 10;249(17):5421–5427. [PubMed] [Google Scholar]
- De Meyts P., Roth J. Cooperativity in ligand binding: a new graphic analysis. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1118–1126. doi: 10.1016/0006-291x(75)90473-8. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., Mendlinger S., Fertig J. W. A simple method to determine fat cell size and number in four mammalian species. Am J Physiol. 1971 Sep;221(3):850–858. doi: 10.1152/ajplegacy.1971.221.3.850. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., Rudman D. Variations in glucose metabolism and sensitivity to insulin of the rat's adipose tissue, in relation to age and body weight. Endocrinology. 1968 Jun;82(6):1133–1141. doi: 10.1210/endo-82-6-1133. [DOI] [PubMed] [Google Scholar]
- Freychet P. Interactions polypeptide hormones with cell membrane specific receptors: studies with insulin and glucagon. Diabetologia. 1976 May;12(2):83–100. doi: 10.1007/BF00428972. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta. 1973 Aug 17;320(1):16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Gliemann J., Osterlind K., Vinten J., Gammeltoft S. A procedure for measurement of distribution spaces in isolated fat cells. Biochim Biophys Acta. 1972 Nov 24;286(1):1–9. doi: 10.1016/0304-4165(72)90082-7. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974 Apr 29;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. I. A functional change in the plasma membrane associated with the rate of cell growth. J Biol Chem. 1974 Jun 10;249(11):3366–3374. [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Olefsky J. M. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1975 Dec;56(6):1499–1508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M., Jen P., Reaven G. M., Alto P. Insulin binding to isolated human adipocytes. Diabetes. 1974 Jul;23(7):565–571. doi: 10.2337/diab.23.7.565. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Johnson J., Liu F., Jen P., Reaven G. M. The effects of acute and chronic dexamethasone administration on insulin binding to isolated rat hepatocytes and adipocytes. Metabolism. 1975 Apr;24(4):517–527. doi: 10.1016/0026-0495(75)90076-1. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Reaven G. M. Effects of age and obesity on insulin binding to isolated adipocytes. Endocrinology. 1975 Jun;96(6):1486–1498. doi: 10.1210/endo-96-6-1486. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M. The effects of spontaneous obesity on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J Clin Invest. 1976 Apr;57(4):842–851. doi: 10.1172/JCI108360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J., Reaven G. M. The human lymphocyte: a model for the study of insulin-receptor interaction. J Clin Endocrinol Metab. 1974 Apr;38(4):554–560. doi: 10.1210/jcem-38-4-554. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Renner E. D., Plagemann P. G., Bernlohr R. W. Permeation of glucose by simple and facilitated diffusion by Novikoff rat hepatoma cells in suspension culture and its relationship to glucose metabolism. J Biol Chem. 1972 Sep 25;247(18):5765–5776. [PubMed] [Google Scholar]
- Soll A. H., Goldfine I. D., Roth J., Kahn C. R. Thymic lymphocytes in obese (ob-ob) mice. A mirror of the insulin receptor defect in liver and fat. J Biol Chem. 1974 Jul 10;249(13):4127–4131. [PubMed] [Google Scholar]
- WICK A. N., DRURY D. R., NAKADA H. I., WOLFE J. B. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem. 1957 Feb;224(2):963–969. [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]


