Abstract
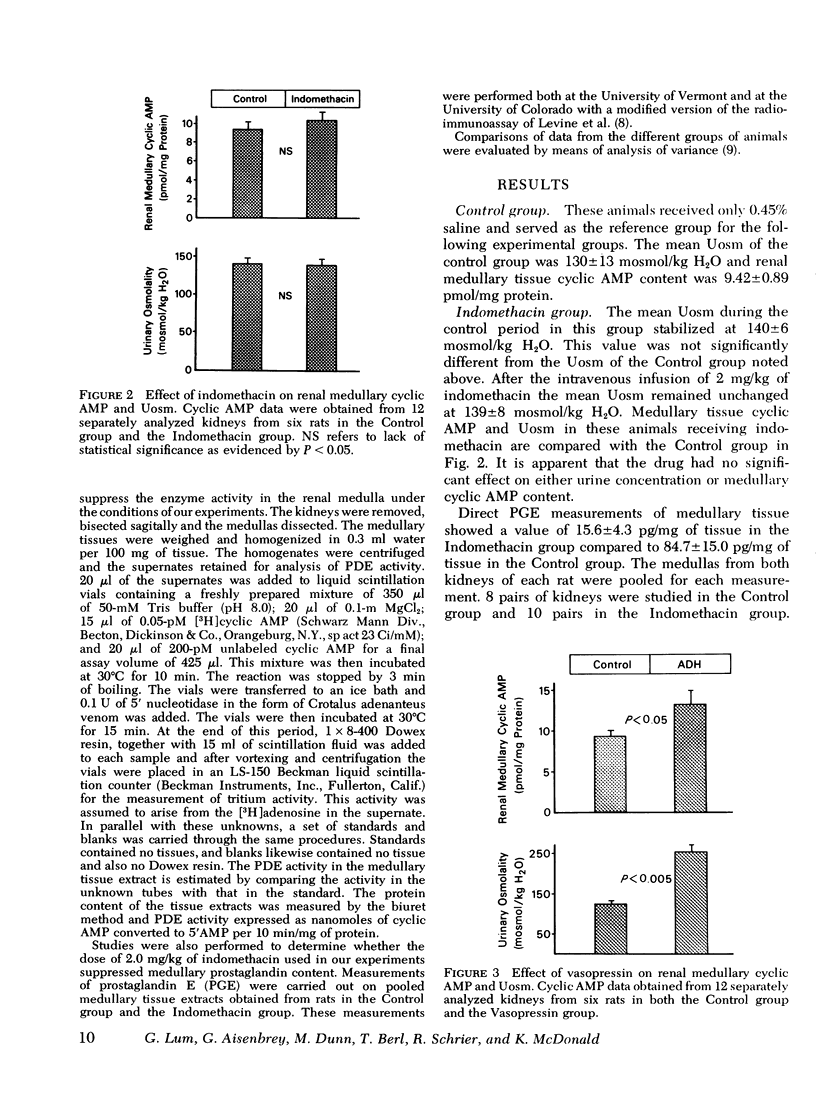
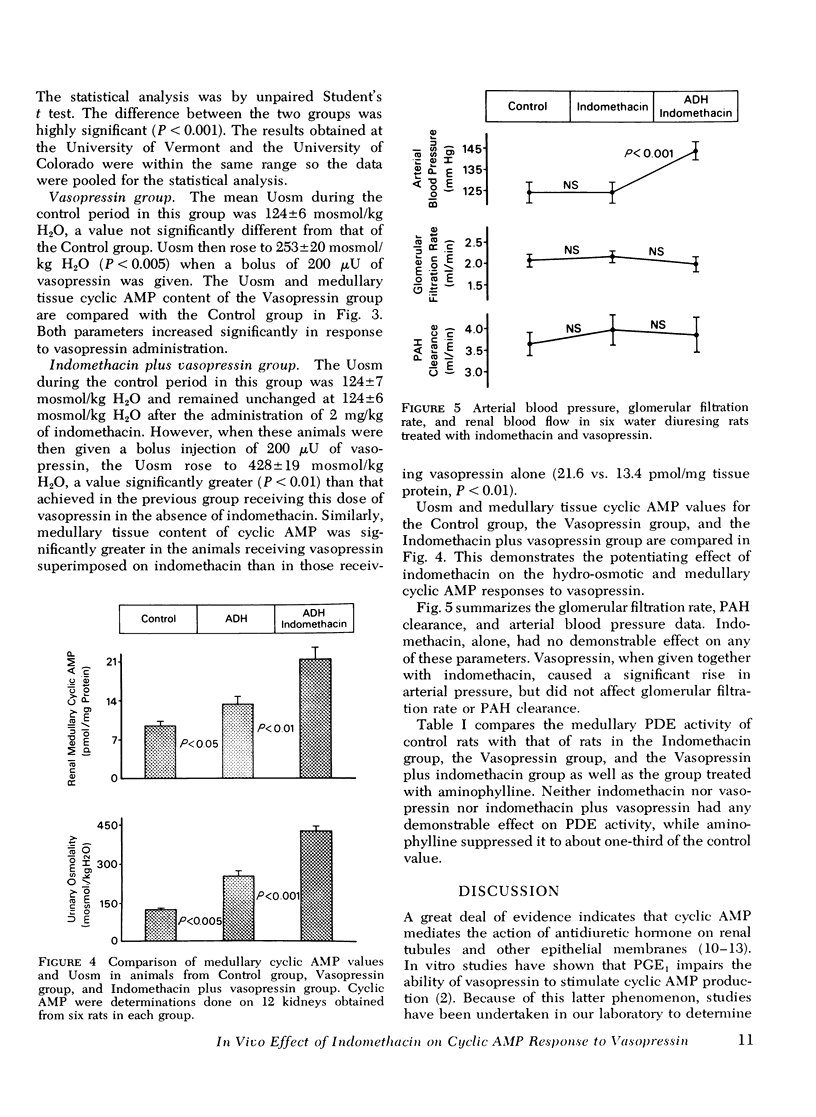
In a previous study we demonstrated that indomethacin potentiated the hydro-osmotic action of vasopressin in vivo. It was hypothesized that this action of indomethacin was due to its ability to suppress renal medullary prostaglandin synthesis, since in vitro studies have suggested that prostaglandins interfere with the ability of vasopressin to stimulate production of its intracellular mediator, cyclic AMP. In the present study this hypothesis was tested in vivo. Anesthetized rats undergoing a water diuresis were studied. In a control group, bolus injections of 200 muU of vasopressin caused a rise in urinary osmolality (Uosm) from 124 +/- 6 to 253 +/- 20 mosmol/kg H2O (P less than 0.005). In a group treated with 2 mg/kg of indomethacin the same dose of vasopressin caused a significantly greater (P less than 0.001) rise in Uosm from 124 +/- 7 to 428 +/- 19 mosmol/kg H2O. Medullary tissue cyclic AMP rose from 9.4 +/- 0.9 to 13.4 +/- 1.7 (P less than 0.05) pmol/mg tissue protein after vasopressin administration in animals receiving no indomethacin, while in indomethacin-treated animals there was a significantly greater rise (P less than 0.001) in medullary cyclic AMP from 10.4 +/- 0.9 to 21.6 +/- 2.1 pmol/mg tissue protein in response to the vasopressin injections. In neither control animals nor indomethacin-treated animals were there significant changes in renal hemodynamics, as measured by clearance techniques. Indomethacin, when given alone, had no effect on Uosm or medullary tissue cyclic AMP. Indomethacin did, however, reduce medullary prostaglandin E content from 84.7 +/- 15.0 to 15.6 +/- 4.3 pg/mg tissue. This study has shown that indomethacin, in a dose which suppresses medullary prostaglandin content, potentiates the ability of vasopressin to increase the tissue content of its intracellular mediator, cyclic AMP. Indomethacin caused no demonstrable inhibition of cyclic AMP phosphodiesterase. Therefore, it seems likely that indomethacin enhanced the ability of vasopressin to increase medullary cyclic AMP levels by causing an increased production rather than decreased destruction of the nucleotide. We conclude that this action of indomethacin contributes to its ability to potentiate the hydro-osmotic action of vasopressin in vivo. A corollary to this conclusion is that endogenous medullary prostaglandin E's may be significant physiological modulators of the renal response to vasopressin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. J., Berl T., McDonald K. D., Schrier R. W. Evidence for an in vivo antagonism between vasopressin and prostaglandin in the mammalian kidney. J Clin Invest. 1975 Aug;56(2):420–426. doi: 10.1172/JCI108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck N. P., Kaneko T., Zor U., Field J. B., Davis B. B. Effects of vasopressin and prostaglandin E 1 on the adenyl cyclase-cyclic 3',5'-adenosine monophosphate system of the renal medulla of the rat. J Clin Invest. 1971 Dec;50(12):2461–2465. doi: 10.1172/JCI106746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker G., Thomas L. J., Jr, Appleman M. M. The assay of adenosine 3',5'-cyclic monophosphate and guanosine 3',5'-cyclic monophosphate in biological materials by enzymatic radioisotopic displacement. Biochemistry. 1968 Dec;7(12):4177–4181. doi: 10.1021/bi00852a006. [DOI] [PubMed] [Google Scholar]
- Chase L. R., Aurbach G. D. Renal adenyl cyclase: anatomically separate sites for parathyroid hormone and vasopressin. Science. 1968 Feb 2;159(3814):545–547. doi: 10.1126/science.159.3814.545. [DOI] [PubMed] [Google Scholar]
- Dousa T. P. Role of cyclic AMP in the action of antidiuretic hormone on kidney. Life Sci. 1973 Oct 16;13(8):1033–1040. doi: 10.1016/0024-3205(73)90371-8. [DOI] [PubMed] [Google Scholar]
- Flores A. G., Sharp G. W. Endogenous prostaglandins and osmotic water flow in the toad bladder. Am J Physiol. 1972 Dec;223(6):1392–1397. doi: 10.1152/ajplegacy.1972.223.6.1392. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Gilman A. G. Protein binding assays for cyclic nucleotides. Adv Cyclic Nucleotide Res. 1972;2:9–24. [PubMed] [Google Scholar]
- Grantham J. J., Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3',5'-monophosphate, and theophylline. J Clin Invest. 1968 May;47(5):1154–1161. doi: 10.1172/JCI105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler J. S., Butcher R. W., Sutherland E. W., Orloff J. The effect of vasopressin and of theophylline on the concentration of adenosine 3',5'-phosphate in the urinary bladder of the toad. J Biol Chem. 1965 Nov;240(11):4524–4526. [PubMed] [Google Scholar]
- Hinman J. W. Prostaglandins. Annu Rev Biochem. 1972;41:161–178. doi: 10.1146/annurev.bi.41.070172.001113. [DOI] [PubMed] [Google Scholar]
- Itskovitz H. D., Stemper J., Pacholczyk D., McGiff J. C. Renal prostaglandins: determinants of intrarenal distribution of blood flow in the dog. Clin Sci Mol Med Suppl. 1973 Aug;45 (Suppl 1):321s–3214. doi: 10.1042/cs045321s. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum M. A., White N., Stein J. H., Ferris T. F. Redistribution of renal cortical blood flow during inhibition of prostaglandin synthesis. Am J Physiol. 1974 Oct;227(4):801–805. doi: 10.1152/ajplegacy.1974.227.4.801. [DOI] [PubMed] [Google Scholar]
- Levine L., Gjtierrez Cernosek R. M., Van Vunakis H. Specificities of prostaglandins B 1 , F 1 , and F 2 antigen-antibody reactions. J Biol Chem. 1971 Nov 25;246(22):6782–6785. [PubMed] [Google Scholar]
- Lipson L. C., Sharp G. W. Effect of prostaglandin E1 on sodium transport and osmotic water flow in the toad bladder. Am J Physiol. 1971 Apr;220(4):1046–1052. doi: 10.1152/ajplegacy.1971.220.4.1046. [DOI] [PubMed] [Google Scholar]
- Newcombe D. S., Thanassi N. M., Ciosek C. P., Jr Cartilage cyclic nucleotide phosphodiesterase: inhibition by anti-inflammatory agents. Life Sci. 1974 Feb 1;14(3):505–519. doi: 10.1016/0024-3205(74)90365-8. [DOI] [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S. The similarity of effects of vasopressin, adenosine-3',5'-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest. 1962 Apr;41:702–709. doi: 10.1172/JCI104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]



