The prevention of degenerative disease through dietary intervention (chemoprevention), either by the recommendation of specific diets or by the use of dietary supplements, has enormous potential for improving human health. This is particularly the case for the prevention of cancer, where current therapeutic approaches are crude or limited. It has been appreciated through history that diet can affect disease pathogenesis and progression. However, it is only in recent times that we have begun to gain an understanding of what the dietary factors are and how they act at a mechanistic level.
There is strong epidemiological evidence that a diet of fruit and vegetables can prevent a range of human cancers (1, 2). This, together with laboratory studies, led to the proposal that the major protective dietary components are antioxidants such as vitamin C, vitamin E, β-carotene, etc.; the specific hypothesis was that these antioxidant free radical scavenging agents protect against the toxic or mutagenic effects of reactive oxygen species generated either endogenously in the body or by exogenous chemicals present in food, water, or air (3).
The seminal work from the laboratories of Wattenberg, Talalay, and Conney has profoundly altered our perspectives on this theme. During the 1970s, Wattenberg's group (4) demonstrated that a wide range of nonnutrient dietary chemicals other than those described above, as well as phenolic antioxidants, can profoundly inhibit chemical carcinogenesis in laboratory animals. These effects were ascribed to the ability of these agents to influence both the metabolism and disposition of the carcinogen, and also to enhance the cellular capacity to combat oxidative stress (5–8). Essentially two mechanisms were proposed that involved either the inhibition of carcinogen activation, free radical production and sequestering of reactive oxygen species, or the induction of drug and foreign compound metabolizing enzymes that protect cells from the toxic effects of environmental chemicals.
Focusing on enzyme induction, Talalay's group (9, 10) proceeded during the 1980s to establish that the ability of a foreign compound (xenobiotic) to serve as a Michael reaction acceptor represented an important chemical feature of agents that could act in this manner. In this issue of PNAS, Dinkova-Kostova et al. (11) have made a significant further advance by providing evidence that thiol-reactivity is a key determinant for the activity and potency of these compounds. This finding has important implications, not only in furthering our understanding of how chemopreventive agents act and how to design novel protective agents, but also in identifying the dietary components that may be most efficacious in preventing disease.
An understanding of how such chemicals influence gene expression, identification of the proteins that mediate their effects, and the characterization of the spectrum of toxicities against which they afford protection is critical for the rational application of chemoprevention strategies. In another paper in this issue of PNAS, Ramos-Gomez et al. (12) demonstrate that mice deficient in the bZIP transcription factor, Nrf2, which as a result have a compromised capacity to respond to chemopreventive agents, have increased sensitivity to the carcinogenic effects of the polycyclic aromatic hydrocarbon, benzo(a)pyrene. This demonstrates that this transcription factor is of central importance both in chemical carcinogenesis and chemoprevention.
An Evolutionary Context
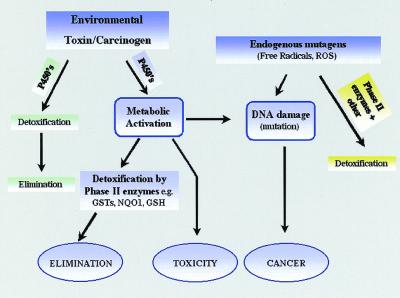
The capacity of organisms to withstand the toxic effects of environmental chemicals is fundamental to their survival. As a consequence, a large number of genes have evolved whose specific role is to remove from the host the toxic threat. The proteins they encode include Phase I and Phase II detoxification enzymes, such as the cytochrome P450-dependent monooxygenases, and a range of glutathione-dependent enzymes including the glutathione S-transferases (13). The primary function of cytochrome P450 enzymes is to insert an atom of molecular oxygen into the substrate, resulting in most cases in increased hydrophilicity and elimination. However, this reaction can also result in the activation of the chemical to a toxic or mutagenic product. This is the major pathway of chemical carcinogenesis (Fig. 1). Such products are normally detoxified by a range of Phase II enzymes including quinone reductase and the glutathione S-transferases; in the latter case through formation of glutathione conjugates (14). Certain of these proteins can also act as peroxidases and therefore prevent the deleterious effects of oxidative stress. Both the cytochrome P450 and glutathione S-transferase systems are multigene families of proteins with each enzyme exhibiting unique, but in some cases overlapping, substrate specificity.
Figure 1.
Pathways of chemical toxicity. GSTs, glutathione S-transferase; NQO1, quinone reductase; GSH, reduced glutathione; P450's, cytochrome P450s; ROS, reactive oxygen species.
It is known from the 1950s work of Conney et al. (15) that these enzymes provide an adaptive response to environmental challenge and that many of them are inducible. Transcription of Phase I and Phase II carcinogen-metabolizing genes can be increased dramatically by exposure to certain chemical agents leading to profound changes in detoxification capacity and sensitivity to carcinogens (16). Furthermore, the enzyme γ-glutamylcysteine synthase, which catalyzes the rate-limiting step in the synthesis of the endogenous antioxidant glutathione, is also inducible. The capacity of chemicals or dietary factors to prevent carcinogenesis has been shown to involve both inhibition of metabolic activation by the P450 system or the induction of chemical detoxification and antioxidant defense. As Ramos-Gomez et al. point out in this issue (12), chemopreventive agents that act as enzyme inducers rather than cytochrome P450 inhibitors or simply as radical scavengers are attractive because they are likely to be more potent and the duration of their protective effects will probably be longer lasting.
Over the last few years there has been a significant increase in our knowledge of the pathways that regulate the expression of these genes (17). It is assumed that the presence of a number of regulatory mechanisms reflects the need to respond to different classes of environmental cytotoxins. The resultant increase in gene expression leads to increased detoxification capacity. This implies that the naturally occurring inducing agents are themselves potentially cytotoxic or that they mimic such species. The generation of cytotoxic molecules, phytoallexins, by plants as part of their natural defense against parasites and predators rationalized the need for these adaptive response systems in organisms that eat plants (17). Indeed, many of the chemicals produced by plants for self protection are those that regulate the adaptive response system in mammals.
Adaptive Response Systems to Environmental Challenge
The above discussion gives some credence to the adage, “A little of what is bad for you is good for you.” It is becoming clear that potentially toxic molecules that activate mammalian detoxification pathways can have a profound effect on subsequent host sensitivity to other toxins, or to carcinogens. The pathways by which cells sense and adapt to the presence of chemicals are numerous and complex. A number of transcription factors have been implicated in mediating these adaptive response processes (Table 1). Of particular importance and relevance to chemoprevention and to the papers in this issue of PNAS has been the finding that the induction of a range of Phase II detoxification enzymes (including the glutathione S-transferases, quinone reductase, and aldo-keto reductase), as well as proteins involved in the regulation of glutathione itself (18), are regulated through a common element, termed the antioxidant response element [ARE (EpRE)], in their gene promoters (19). Subsequently, it was pointed out that bZIP transcription factors including Nrf1 and Nrf2, first isolated as proteins involved in the regulation of globin genes, can bind to this motif (20, 21).
Table 1.
Transcription factors mediating the regulation of cytoprotective genes
| Factor | Induced enzymes |
|---|---|
| Ah receptor | CYP1A, CYP1B, GSTA, NQO1, UGT |
| PXR | CYP3A |
| PPAR α | CYP4A, UGTs |
| CAR | CYP2B, CYP2C, GSTA, GSTM |
| Nrf2/Nrf1 | GST, HO-1, NQO1, GCS |
| AP1 | NQO1, GSTP1 |
CYP, cytochrome P450—numbers and letters represent family and subfamily, respectively; GST, glutathione S-transferase—letters represent family; HO-1, hemeoxygenase 1; NQO1, quinone reductase; UGT, glucuronyl transferase; GCS, gamma glutamyl cysteine synthase.
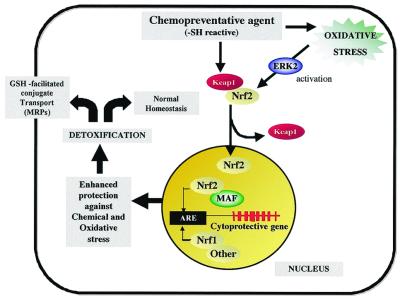
In an elegant study, Yamamoto and colleagues (22) demonstrated that, similar to NFκB, under normal conditions Nrf2 is anchored in the cytoplasm by Keap I. Exposure of cells to either oxidative stress or a chemopreventive agent dissociates this complex resulting in the translocation of Nrf2 to the nucleus (Fig. 2). Nrf2 then heterodimerizes with members of the small Maf family of transcription factors to activate transcription through the ARE. In addition to Nrf2, Nrf1 and possibly other transcription factors can interact with the ARE element to either activate or repress its function.
Figure 2.
Role of the antioxidant response element and the transcription factor Nrf2 in chemoprevention. ARE, antioxidant response element; Keap1, Kelch-like ECH associated protein 1, where ECH is chicken Nrf2; Nrf2, NF-E2 related factor; ERK2, extracellular regulated kinase 2, a component of the MAP kinase pathway postulated to regulate Nrf2; MRP, multidrug resistance related protein; MAF, small Maf proteins (MafK).
How is the Nrf2/Keap I Complex Activated?
The dissociation of Nrf2 from Keap I may involve modification of either one of these proteins, and could be achieved by direct or indirect mechanisms. For example, Nrf2 can be phosphorylated by components of the MAP kinase cascade (23), which could result in its dissociation. Therefore, stimulation of such pathways by toxic stress will result in Nrf2 activation. The paper study by Dinkova-Kostova et al. (11) provides an alternative and exciting possibility that the dissociation of this complex may be potentiated by the direct interaction of the chemoprotective agents with reactive thiol residues in either of the two proteins. This hypothesis is supported by the strong relationship between the potency of the agents as inducers of gene expression through the ARE and their rate of reaction with sulfhydryl groups. This mechanism implies that the inducing agent will become covalently bound either to Keap I or Nrf2. It will be important to establish whether this is the case and to identify the specific cysteine residues involved. It remains feasible that the chemopreventive agent reacts with an alternative target, which in turn activates this system (24). There are now several studies demonstrating the role of Nrf2 in both the constitutive and inducible expression of many Phase II detoxification enzymes in the liver and in other tissues (23, 25). This was also an important finding in the paper of Ramos-Gomez et al. (12). It should be noted, however, that at present there is no satisfactory definition of constitutive expression of proteins regulated through the ARE. Constitutive expression could either reflect exposure of the animals to chemicals in the diet that activate this system or the activity of endogenous inducers such as oxidative stress. In any event, it appears that this system does play a pivotal role in chemoprevention.
Functional Characterization of the ARE System
The use of Nrf2 null mice generated by Itoh et al. (25) and Chan & Kan (27) provide a powerful tool for studying the role of this transcription factor in chemoprevention. It has been shown that mice deficient in Nrf2 are apparently normal, but have increased sensitivity to the toxic effects of certain chemopreventive agents such as butylated hydroxytoluene (27), butylated hydroxyanisole, and ethoxyquin (M. McMahon, J. D. Hayes, C. J. Henderson, and C.R.W., unpublished data). These data support the hypothesis that this system has evolved to protect against dietary cytotoxins.
The work of Ramos-Gomez et al. (12) takes these observations an important step further by providing a demonstration that this factor determines, at least in part, sensitivity to the carcinogenic effects of benzo(a)pyrene. They also show that Nrf2 mediates the ability of the chemopreventive agent, oltipraz, to reduce benzo(a)pyrene carcinogenicity. These findings have a number of implications and raise a number of questions. Of particular importance is that they mechanistically identify Nrf2 as an important transcription factor involved in chemoprevention in humans and lead the way to establishing its role in carcinogenesis induced by other agents, both at the level of tumor initiation and promotion. The observation that tumor incidence was increased in Nrf2 null animals not treated with a chemopreventive agent could be interpreted as due either to the effects on the basal expression of detoxification enzymes or on tumor progression caused by endogenous factors. In this regard it will be important to evaluate whether the null mice exhibit increased sensitivity to carcinogenesis in models where exposure to exogenous mutagens is not required.
Prospects for Chemoprevention
Despite its enormous potential, research in many countries on cancer chemoprevention, and chemoprevention of disease in general, has received little attention or financial support relative to the development of new cancer treatments. This short-sighted view belies the extraordinary advances being made in understanding how chemical agents can prevent human disease. Such advances are reflected in the two papers published in this issue of PNAS and point the way for further important studies. Optimal chemopreventive agents will need to be potent and long lasting (i.e., without the need for continuous administration and devoid of side effects or risk of toxicity).
The work of Dinkova-Kostova et al. (11) and previous work of the Talalay group (5, 7, 9, 10) is providing increasing insight into the structural elements required to optimize chemopreventive agents. These studies need to be extended and further refined to the degree that the balance between the beneficial versus potentially harmful effects of particular agents can be established and the agents that are beneficial in our diet predicted. It remains to be established how these agents act and to rationalize their capacity to react with intracellular protein targets before they are sequestered by other intracellular thiols such as glutathione. The potential for developing chemopreventive agents that work through induction of cytoprotective genes rather than simply as direct acting antioxidants is extremely attractive.
With this in mind there are already clinical trials in place evaluating such agents, and some evidence has been reported that the Nrf2 system is active in humans in vivo (28). In future studies it will be of central importance to build on the paper of Ramos-Gomez et al. (12) to establish which classes of chemicals and in which tissues the Nrf2 system will afford protection, either with or without exposure to a chemopreventive agent. In the long term, agents need to be developed with the necessary potency and duration of action and toxicology so that they can be applied to healthy human populations for the prevention of human disease. Exploitation of the Nrf2/ARE system may provide one mechanism for achieving this.
Acknowledgments
I thank Professor J. D. Hayes and Dr. C. R. Elcombe for critically reading this manuscript and Dr. M. Chamberlain for preparing the figures.
Footnotes
References
- 1.Potter J D, editor. Food, Nutrition and the Prevention of Cancer: A global perspective. Washington, D.C.: World Cancer Research Fund/American Institute for Cancer Research; 1997. [Google Scholar]
- 2.Steinmetz K A, Potter J D. Cancer Causes Control. 1991;2:325–357. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- 3.Ames B N, Magaw R, Gold L S. Science. 1987;236:271–280. doi: 10.1126/science.3563506. [DOI] [PubMed] [Google Scholar]
- 4.Wattenberg L W. Adv Cancer Res. 1978;26:197–226. doi: 10.1016/s0065-230x(08)60088-3. [DOI] [PubMed] [Google Scholar]
- 5.Benson A M, Batzinger R P, Ou S-YL, Bueding E, Cha Y-N, Talalay P. Cancer Res. 1978;38:4486–4495. [PubMed] [Google Scholar]
- 6.Pantuck E J, Pantuck C B, Garland W A, Min B H, Wattenberg L W, Anderson K E, Kappas A, Conney A H. Clin Pharmacol Ther. 1978;25:88–95. doi: 10.1002/cpt197925188. [DOI] [PubMed] [Google Scholar]
- 7.Benson A M, Hunkeler M J, Talalay P. Proc Natl Acad Sci USA. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wattenberg L W. Cancer Res. 1985;45:1–8. [PubMed] [Google Scholar]
- 9.Talalay P, DeLong M J, Prochaska H J. Proc Natl Acad Sci USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinkova-Kostova A T, Talalay P. Free Radical Biol Med. 2000;29:231–240. doi: 10.1016/s0891-5849(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 11.Dinkova-Kostova A T, Massiah M A, Bozak R E, Hicks R J, Talalay P. Proc Natl Acad Sci USA. 2001;98:3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-Gomez M, Kwak M-K, Dolan P M, Itoh K, Yamamoto M, Talalay P, Kensler T W. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf C R, Mahmood A, Henderson C J, McLeod R, Manson M M, Neal G E, Hayes J D. In: Principles of Chemoprevention. Stewart BW, McGregor D, Kleihues P, editors. Lyon, France: International Agency for Research on Cancer; 1996. pp. 165–173. [Google Scholar]
- 14.Hayes J D, Pulford D J. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 15.Conney A H, Mueller G C, Millar J A. Cancer Res. 1956;16:450–459. [PubMed] [Google Scholar]
- 16.Wolf C R. Cancer Surv. 1990;9:437–474. [PubMed] [Google Scholar]
- 17.Ames B N. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 18.Hayes J D, Ellis E M, Neal G E, Harrison D J, Manson M M. Biochem Soc Symp. 1999;64:141–168. [PubMed] [Google Scholar]
- 19.Rushmore T H, King R G, Paulson K E, Pickett C B. Proc Natl Acad Sci USA. 1990;87:3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerppola T K, Curran T. Oncogene. 1994;9:3149–3158. [PubMed] [Google Scholar]
- 21.Venugopal R, Jaiswal A K. Proc Natl Acad Sci USA. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel J D, Yamamoto M. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu R, Lei W, Mandlekar S, Weber M J, Der C J, Wu J, Kong A-N T. J Biol Chem. 1999;274:27545–27552. doi: 10.1074/jbc.274.39.27545. [DOI] [PubMed] [Google Scholar]
- 24.Hayes J D, Chanas S A, Henderson C J, McMahon M, Sun C, Moffat G J, Wolf C R, Yamamoto M. Biochem Soc Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- 25.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 26.Henderson C J, Smith A G, Ure J, Brown K, Bacon E J, Wolf C R. Proc Natl Acad Sci USA. 1998;95:5275–5280. doi: 10.1073/pnas.95.9.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan K, Kan Y W. Proc Natl Acad Sci USA. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J-S, Shen X, He X, Zhu Y-R, Zhang B-C, Wang J-B, Qiang G-S, Kuang S-Y, Zarba A, Egner P A, et al. J Natl Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]