Abstract
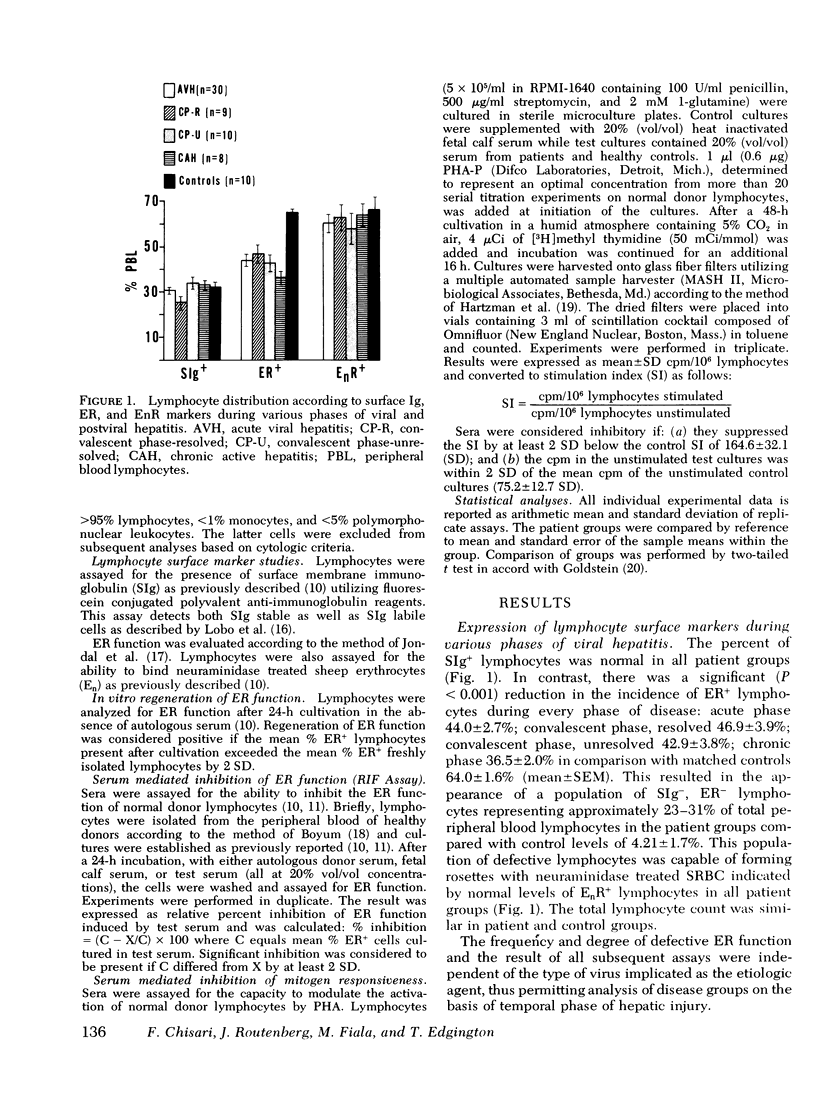
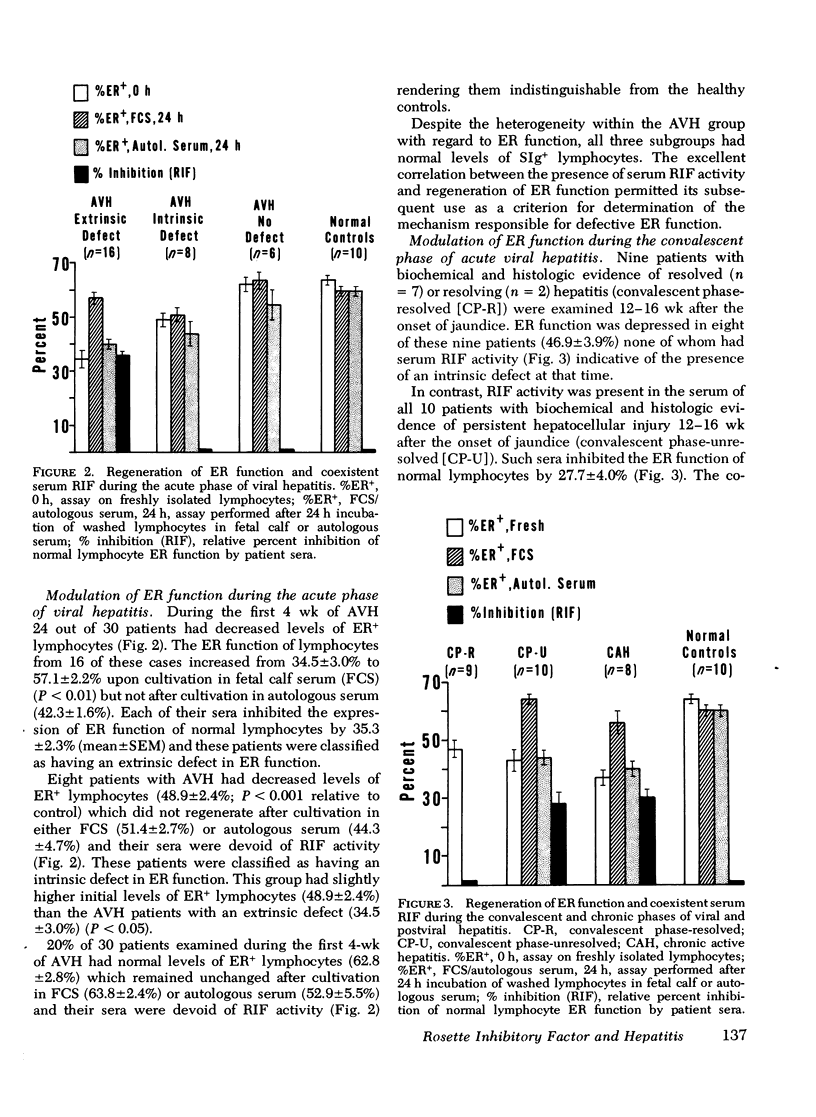
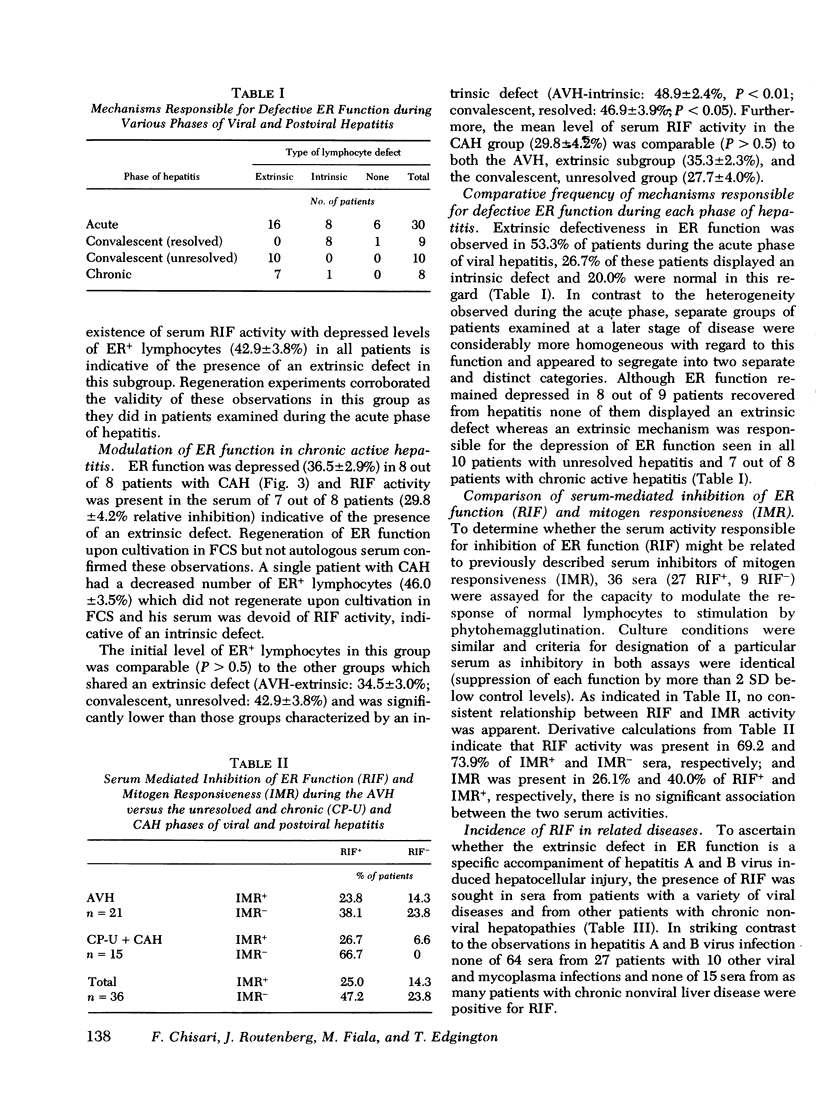
Defective T-lymphocyte E rosette (ER) function associated with viral hepatitis A and B may be due to mechanisms extrinsic or intrinsic to the target lymphocyte. The extrinsic defect is induced by an immunoregulatory plasma lipoprotein (RIF) and has the capacity to regenerate ER function in vitro. The intrinsic defect is refractory to regeneration and is not associated with RIF. Although both mechanisms occur with high frequency during the acute phase of viral hepatitis they tend to segregate in accordance with progression of hepatocellular injury at later stages of the disease. The extrinsic defect was observed in 7 out of 8 patients with longstanding chronic active hepatitis and in 10 out of 10 patients with unresolved hepatitis 12 wk after the onset of jaundice. In contrast, none of nine patients with resolved hepatitis had extrinsically defective ER function 12 wk after the onset of jaundice whereas eight of them displayed an intrinsic defect of ER function at that time. Among the various viral and liver diseases studied RIF appeared to be specific for hepatitis A and B viral infections. None of 64 sera from a variety of viral infections including Epstein-Barr virus cytomegalovirus mononucleosis with associated hepatitis nor 15 sera from patients with several chronic nonviral liver diseases were positive for RIF. RIF and its associated extrinsic defect in ER function therefore appear to correlate with a particular type of hepatocellular injury initiated by the hepatitis A and B viruses that may have a propensity for persistence and(or) progression to an aggressive form of chronic hepatitis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentwich Z., Douglas S. D., Siegal F. P., Kunkel H. G. Human lymphocyte-sheep erythrocyte rosette formation: some characteristics of the interaction. Clin Immunol Immunopathol. 1973 Jul;1(4):511–522. doi: 10.1016/0090-1229(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Bernstein I. M., Webster K. H., Williams R. C., Jr, Strickland R. G. Reduction in circulating T lymphocytes in alcoholic liver disease. Lancet. 1974 Aug 31;2(7879):488–490. doi: 10.1016/s0140-6736(74)92015-7. [DOI] [PubMed] [Google Scholar]
- Chisari F. V., Edgington T. S. Human T lymphocyte "E" rosette function. I. A process modulated by intracellular cyclic AMP. J Exp Med. 1974 Oct 1;140(4):1122–1126. doi: 10.1084/jem.140.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Edgington T. S. Lymphocyte E rosette inhibitory factor: a regulatory serum lipoprotein. J Exp Med. 1975 Nov 1;142(5):1092–1107. doi: 10.1084/jem.142.5.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Routenberg J. A., Edgington T. S. Mechanisms responsible for defective human T-lymphocyte sheep erythrocyte rosette function associated with hepatitis B virus infections. J Clin Invest. 1976 May;57(5):1227–1238. doi: 10.1172/JCI108391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss L. K., Edgington T. S. Regulatory serum lipoproteins: regulation of lymphocyte stimulation by a species of low density lipoprotein. J Immunol. 1976 May;116(5):1452–1458. [PubMed] [Google Scholar]
- De Groote J., Desmet V. J., Gedigk P., Korb G., Popper H., Poulsen H., Scheuer P. J., Schmid M., Thaler H., Uehlinger E. A classification of chronic hepatitis. Lancet. 1968 Sep 14;2(7568):626–628. doi: 10.1016/s0140-6736(68)90710-1. [DOI] [PubMed] [Google Scholar]
- DeHoratius R. J., Strickland R. G., Williams R. C., Jr T and B lymphocytes in acute and chronic hepatitis. Clin Immunol Immunopathol. 1974 Apr;2(3):353–360. doi: 10.1016/0090-1229(74)90053-1. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L., Routenberg J. A., Purcell R. H., Hooper R. R., Harrison W. O. Foodhandler-associated outbreak of hepatitis type A. An immune electron microscopic study. Ann Intern Med. 1975 Nov;83(5):647–650. doi: 10.7326/0003-4819-83-5-647. [DOI] [PubMed] [Google Scholar]
- Dudley F. J., Fox R. A., Sherlock S. Cellular immunity and hepatitis-associated, Australia antigen liver disease. Lancet. 1972 Apr 1;1(7753):723–726. doi: 10.1016/s0140-6736(72)90234-6. [DOI] [PubMed] [Google Scholar]
- Eddleston A. L., Williams R. Inadequate antibody response to hBAg or suppressor T-cell defect in development of active chronic hepatitis. Lancet. 1974 Dec 28;2(7896):1543–1545. doi: 10.1016/s0140-6736(74)90287-6. [DOI] [PubMed] [Google Scholar]
- Edgington T. S., Chisari F. V. Immunological aspects of hepatitis B virus infection. Am J Med Sci. 1975 Sep-Oct;270(2):212–227. [PubMed] [Google Scholar]
- Fiala M., Payne J. E., Berne T. V., Moore T. C., Henle W., Montgomerie J. Z., Chatterjee S. N., Guze L. B. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis. 1975 Oct;132(4):421–433. doi: 10.1093/infdis/132.4.421. [DOI] [PubMed] [Google Scholar]
- Galili U., Schlesinger M. The formation of stable E rosettes after neuraminidase treatment of either human peripheral blood lymphocytes or of sheep red blood cells. J Immunol. 1974 May;112(5):1628–1634. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Hartzman R. J., Bach M. L., Bach F. H., Thurman G. B., Sell K. W. Precipitation of radioactively labeled samples: a semi-automatic multiple-sample processor. Cell Immunol. 1972 Jun;4(2):182–186. doi: 10.1016/0008-8749(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey J. H., Hom D. J., Buttrick P. Human T lymphocyte receptors for sheep erythrocytes: conditions for binding including inhibition by cytochalasin B. J Immunol. 1974 Feb;112(2):862–865. [PubMed] [Google Scholar]
- Lobo P. I., Westervelt F. B., Horwitz D. A. Identification of two populations of immunoglobulin-bearing lymphocytes in man. J Immunol. 1975 Jan;114(1 Pt 1):116–119. [PubMed] [Google Scholar]
- Messner R. P., Lindström F. D., Williams R. C., Jr Peripheral blood lymphocyte cell surface markers during the course of systemic lupus erythematosus. J Clin Invest. 1973 Dec;52(12):3046–3056. doi: 10.1172/JCI107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Newberry W. M., Shorey J. W., Sanford J. P., Combes B. Depression of lymphocyte reactivity to phytohemagglutinin by serum from patients with liver disease. Cell Immunol. 1973 Jan;6(1):87–97. doi: 10.1016/0008-8749(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Owen F. L., Fanger M. W. Studies on the human T lymphocyte population. III. Synthesis and release of the lymphocyte receptor for sheep red blood cells by stimulated human T lymphoblasts. J Immunol. 1975 Sep;115(3):765–770. [PubMed] [Google Scholar]
- Parker C. W., Sullivan T. J., Wedner H. J. Cyclic AMP and the immune response;. Adv Cyclic Nucleotide Res. 1974;4(0):1–79. [PubMed] [Google Scholar]
- Peters R. L. Viral hepatitis: a pathologic spectrum. Am J Med Sci. 1975 Jul-Aug;270(1):17–31. doi: 10.1097/00000441-197507000-00004. [DOI] [PubMed] [Google Scholar]
- Popper H., Mackay I. R. Relation between Australia antigen and autoimmune hepatitis. Lancet. 1972 May 27;1(7761):1161–1164. doi: 10.1016/s0140-6736(72)91379-7. [DOI] [PubMed] [Google Scholar]
- Redeker A. G. Viral hepatitis: clinical aspects. Am J Med Sci. 1975 Jul-Aug;270(1):9–16. doi: 10.1097/00000441-197507000-00003. [DOI] [PubMed] [Google Scholar]
- Scheinberg M., Blacklow N. R., Goldstein A. L., Parrino T. A., Rose F. B., Cathcart E. S. Influenza: response of T-cell lymphopenia to thymosin. N Engl J Med. 1976 May 27;294(22):1208–1211. doi: 10.1056/NEJM197605272942204. [DOI] [PubMed] [Google Scholar]
- Schumacher K., Maerker-Alzer G., Wehmer U. A lymphocyte-inhibiting factor isolated from normal human liver. Nature. 1974 Oct 18;251(5476):655–656. doi: 10.1038/251655a0. [DOI] [PubMed] [Google Scholar]
- Takada A., Takada Y., Minowada J. Immunological functions of human T-lymphoid cell line (MOLT). I. Release of immunosuppressive factors from the mixture of MOLT-4 cells and sheep red blood cells. J Exp Med. 1974 Aug 1;140(2):538–548. doi: 10.1084/jem.140.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. E., Incefy G. S., Good R. A. Differentiation of population of peripheral blood lymphocytes into cells bearing sheep erythrocyte receptors in vitro by human thymic extract. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1175–1178. doi: 10.1073/pnas.72.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wands J. R., Perrotto J. L., Alpert E., Isselbacher K. J. Cell-mediated immunity in acute and chronic hepatitis. J Clin Invest. 1975 May;55(5):921–929. doi: 10.1172/JCI108021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Fudenberg H. H. Thymus-derived rosette-forming cells in various human disease states: cancer, lymphoma, bacterial and viral infections, and other diseases. J Clin Invest. 1973 May;52(5):1026–1032. doi: 10.1172/JCI107267. [DOI] [PMC free article] [PubMed] [Google Scholar]



