Abstract
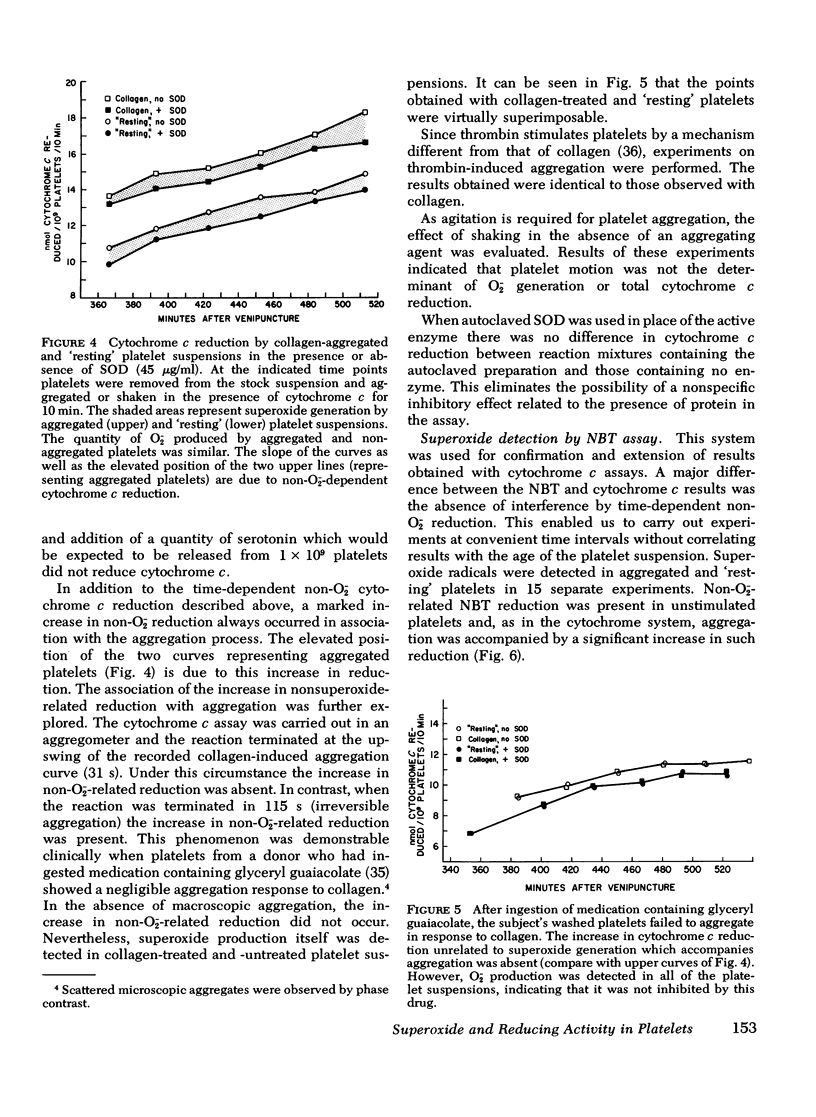
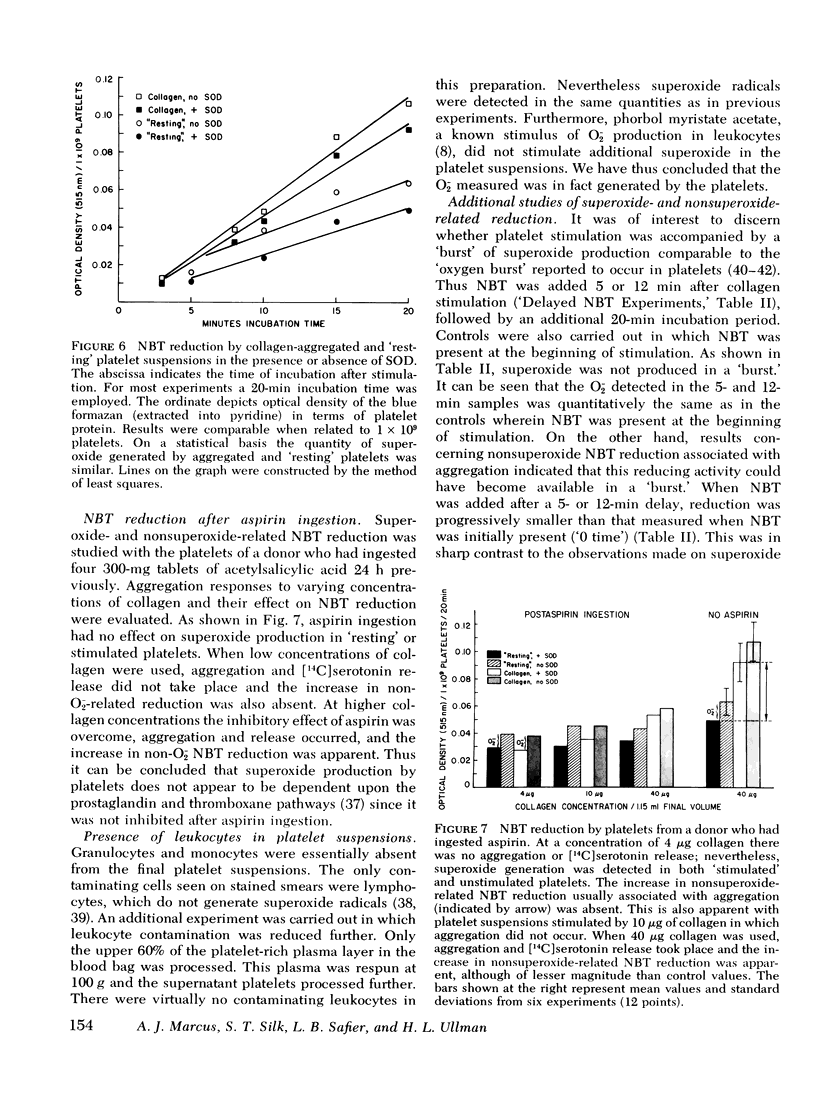
Human platelets contain the cuprozinc (cytoplasmic) and manganese (mitochondrial) forms of superoxide dismutase. Nevertheless, superoxide radicals were detectable in the surrounding medium of metabolically viable platelet suspensions by using two assay systems: cytochrome c and nitroblue tetrazolium. The quantity of superoxide generated by platelets (5 X 10(5) superoxide radicals/platelet per 10 min) was constant and did not increase after aggregation by agents such as collagen and thrombin. The superoxide-generating system was present in the supernate of both aggregated and resting platelets and therefore was not platelet-bound. Platelet superoxide production was unaffected by prior ingestion of aspirin, indicating that the prostaglandin and thromboxane pathways were not involved. Both resting and aggregated platelets exhibited a reductive capacity toward cytochrome c and nitroblue tetrazolium which was unrelated to superoxide production. Furthermore, the aggregation process always resulted in a marked increase in this reduction. The nonsuperoxide reduction associated with aggregation was found to be membrane bound and to decrease with an apparent first order reaction rate (k1 = 0.067 min-1). In addition, accumulative, time-dependent nonsuperoxide-related cytochrome c reduction was also detected. Since there is no superoxide dismutase in plasma, the presence of superoxide radicals in the surrounding medium of platelets may have in vitro significance for platelet and leukocyte concentration and storage and in vivo significance for hemostasis, coagulation, and thrombosis. The nonsuperoxide-related reducing activities may represent a biochemical basis for platelet-blood vessel interactions, with particular reference to blood vessel integrity.
Full text
PDF
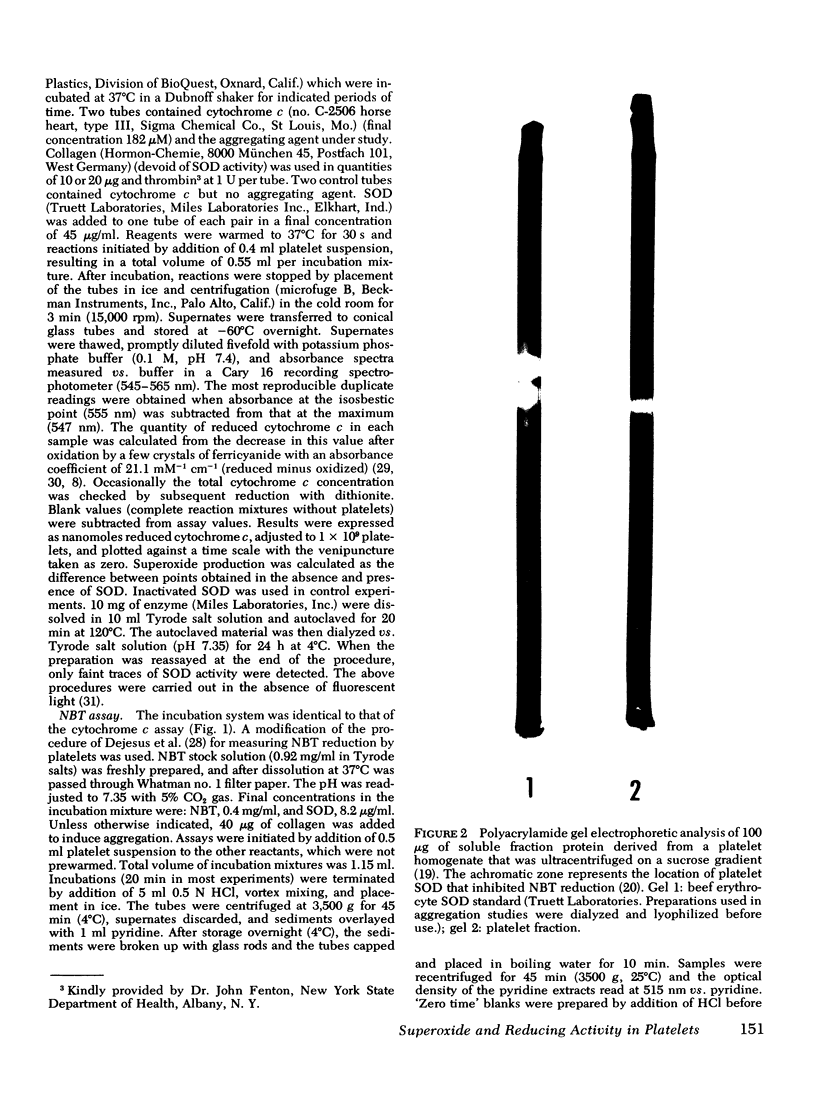





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Ando Y., Steiner M. Distribution of free sulfhydryl and disulfide groups among platelet membrane proteins. Biochim Biophys Acta. 1976 Jan 8;419(1):51–62. doi: 10.1016/0005-2736(76)90371-0. [DOI] [PubMed] [Google Scholar]
- Autor A. P. Reduction of paraquat toxicity by superoxide dismutase. Life Sci. 1974 Apr 1;14(7):1309–1319. doi: 10.1016/0024-3205(74)90439-1. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Murrmann S. K., Davis J., Johnston R. B., Jr The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Invest. 1975 Sep;56(3):571–576. doi: 10.1172/JCI108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Baehner R. L. Subcellular distribution of nitroblue tetrazolium reductase (NBT-R) in human polymorphonuclear leukocytes (PMN). J Lab Clin Med. 1975 Nov;86(5):785–792. [PubMed] [Google Scholar]
- Beauchamp C. O., Fridovich I. Isozymes of superoxide dismutase from wheat germ. Biochim Biophys Acta. 1973 Jul 12;317(1):50–64. doi: 10.1016/0005-2795(73)90198-0. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beckman G., Lundgren E., Tärnvik A. Superoxide dismutase isozymes in different human tissues, their genetic control and intracellular localization. Hum Hered. 1973 Apr;23(4):338–345. doi: 10.1159/000152594. [DOI] [PubMed] [Google Scholar]
- Clawson C. C., White J. G. Platelet interaction with bacteria. II. Fate of the bacteria. Am J Pathol. 1971 Nov;65(2):381–397. [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Kipnes R. S., Babior B. M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N Engl J Med. 1975 Sep 25;293(13):628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- Cushing L. S., Decker W. E., Santos F. K., Schulte T. L., Huber W. Orgotein therapy for inflammation in horses. Mod Vet Pract. 1973 Jul;54(7):17–21. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dejesus M., Jr, Fikrig S., Detwiler T. Phagocytosis-stimulated nitroblue tetrazolium reduction by platelets. J Lab Clin Med. 1972 Jul;80(1):117–124. [PubMed] [Google Scholar]
- Fairshter R. D., Wilson A. F. Paraquat poisoning: manifestations and therapy. Am J Med. 1975 Dec;59(6):751–753. doi: 10.1016/0002-9343(75)90459-3. [DOI] [PubMed] [Google Scholar]
- Fukami M. H., Holmsen H., Bauer J. Thrombin-induced oxygen consumption, malonyldialdehyde formation and serotonin secretion in human platelets. Biochim Biophys Acta. 1976 Mar 25;428(1):253–256. doi: 10.1016/0304-4165(76)90126-4. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Stern A., Peisach J. The mechanism of superoxide anion generation by the interaction of phenylhydrazine with hemoglobin. J Biol Chem. 1976 May 25;251(10):3045–3051. [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeman M. R., Prins H. K. Serotonin uptake and glycolytic activity of human platelets after prolonged incubation with glucose-poor plasma. Haemostasis. 1975;4(2):81–93. doi: 10.1159/000214091. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E. Elaboration of toxic oxygen by-products by neutrophils in a model of immune complex disease. J Clin Invest. 1976 Apr;57(4):836–841. doi: 10.1172/JCI108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug D., Rabani J., Fridovich I. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J Biol Chem. 1972 Aug 10;247(15):4839–4842. [PubMed] [Google Scholar]
- Lindmark D. G., Müller M. Superoxide dismutase in the anaerobic flagellates, Tritrichomonas foetus and Monocercomonas sp. J Biol Chem. 1974 Jul 25;249(14):4634–4637. [PubMed] [Google Scholar]
- Lund-Olesen K., Menander K. B. Orgotein: a new anti-inflammatory metalloprotein drug: preliminary evaluation of clinical efficacy and safety in degenerative joint disease. Curr Ther Res Clin Exp. 1974 Jul;16(7):706–717. [PubMed] [Google Scholar]
- Marberger H., Huber W., Bartsch G., Schulte T., Swoboda P. Orgotein: a new antiinflammatory metalloprotein drug evaluation of clinical efficacy and safety in inflammatory conditions of the urinary tract. Int Urol Nephrol. 1974;6(2):61–74. doi: 10.1007/BF02081999. [DOI] [PubMed] [Google Scholar]
- Marcus A. J. Platelet function. N Engl J Med. 1969 Jun 5;280(23):1278–1284. doi: 10.1056/NEJM196906052802305. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B., Ullman H. L. Interactions between 5-hydroxytryptamine and platelet lipid fractions. Ciba Found Symp. 1975;35:309–326. doi: 10.1002/9780470720172.ch15. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Zucker-Franklin D., Safier L. B., Ullman H. L. Studies on human platelet granules and membranes. J Clin Invest. 1966 Jan;45(1):14–28. doi: 10.1172/JCI105318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. P., Holtzman J. L. The role of catalytic superoxide formation in the O2 inhibition of nitroreductase. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1267–1274. doi: 10.1016/0006-291x(75)90163-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Muenzer J., Weinbach E. C., Wolfe S. M. Oxygen consumption of human blood platelets. I. Effect of thrombin. Biochim Biophys Acta. 1975 Feb 17;376(2):237–242. doi: 10.1016/0005-2728(75)90015-8. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Perry D. W., Ardlie N. G., Packham M. A. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972 Feb;22(2):193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- RAJAGOPALAN K. V., HANDLER P. THE ABSORPTION SPECTRA OF IRON-FLAVOPROTEINS. J Biol Chem. 1964 May;239:1509–1514. [PubMed] [Google Scholar]
- Sagone A. L., Jr, King G. W., Metz E. N. A comparison of the metabolic response to phagocytosis in human granulocytes and monocytes. J Clin Invest. 1976 May;57(5):1352–1358. doi: 10.1172/JCI108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Free radicals and inflammation. Protection of phagocytosine leukocytes by superoxide dismutase. J Clin Invest. 1975 Nov;56(5):1319–1323. doi: 10.1172/JCI108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinet P. M., Lavelle F., Michelson A. M., Jerome H. Superoxide dismutase activities of blood platelets in trisomy 21. Biochem Biophys Res Commun. 1975 Dec 1;67(3):904–909. doi: 10.1016/0006-291x(75)90762-7. [DOI] [PubMed] [Google Scholar]
- Slichter S. J., Harker L. A. Preparation and storage of platelet concentrates. Transfusion. 1976 Jan-Feb;16(1):8–12. doi: 10.1046/j.1537-2995.1976.16176130842.x. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]
- Valdorf-Hansen J. F., Zucker M. B. Effect of temperature and inhibitors on serotonin-14C release from human platelets. Am J Physiol. 1971 Jan;220(1):105–111. doi: 10.1152/ajplegacy.1971.220.1.105. [DOI] [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- Yu C. A., Chiang Y. L., Yu L., King T. E. Photoreduction of cytochrome c1. J Biol Chem. 1975 Aug 25;250(16):6218–6221. [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]