Abstract
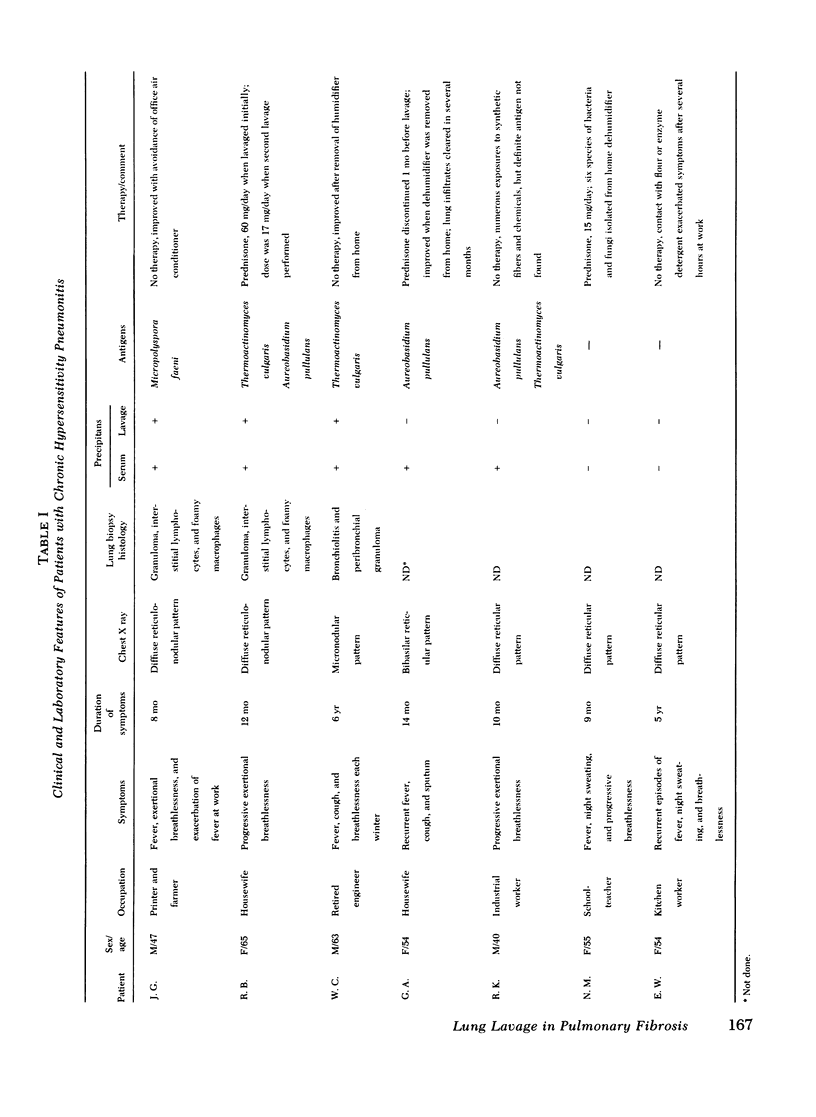
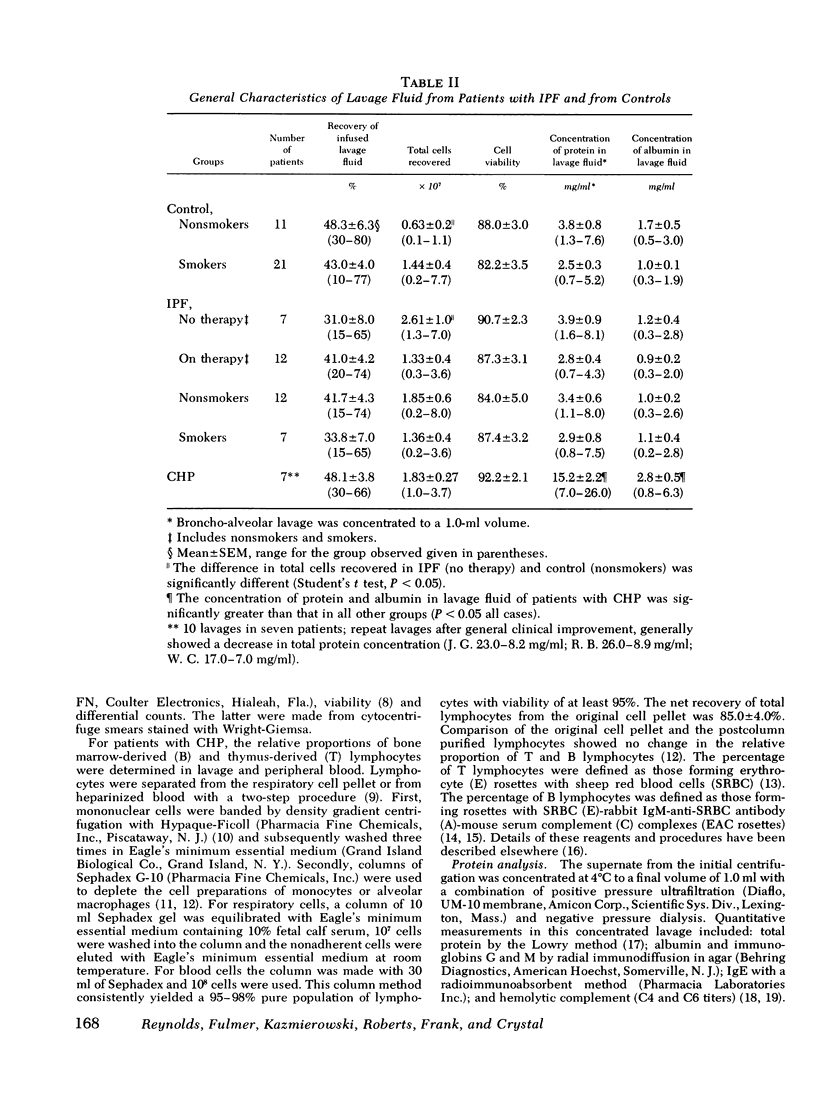
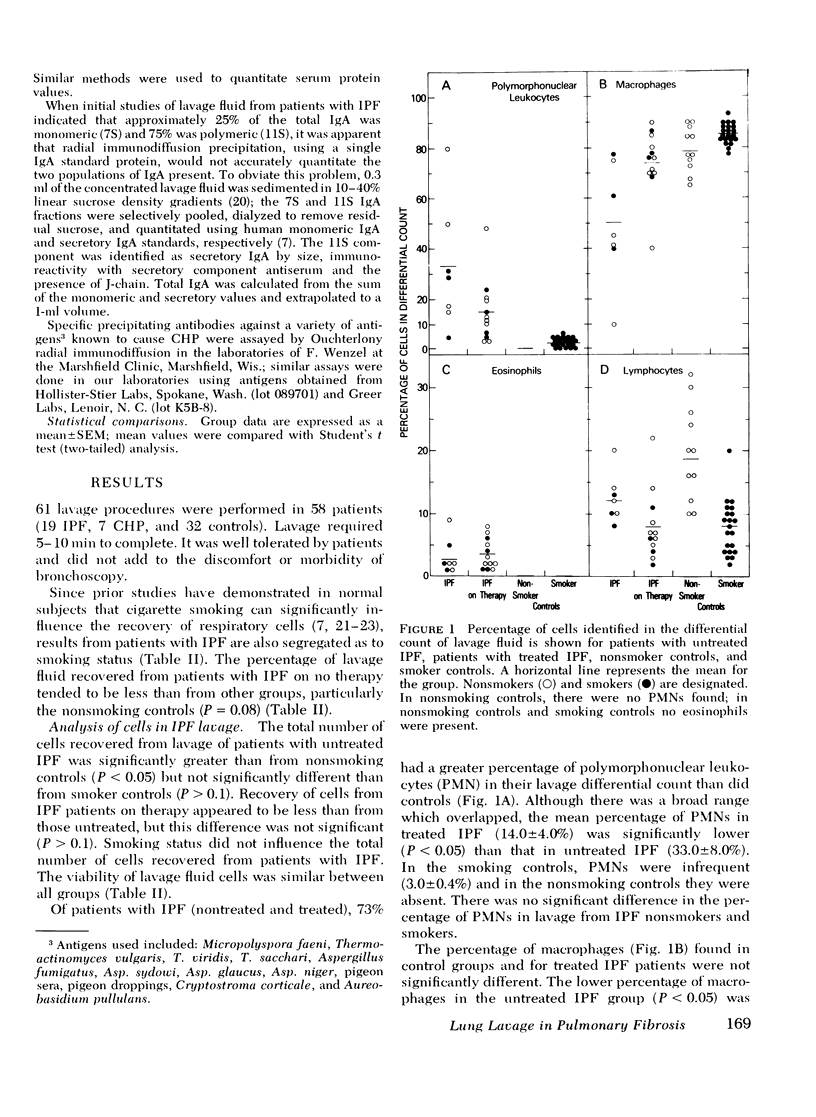
To evaluate cellular and protein components in the lower respiratory tract of patients with idiopathic pulmonary fibrosis (IPF) and chronic hypersensitivity pneumonitis (CHP), limited broncho-alveolar lavage was done in 58 patients (19 IPF, 7 CHP, and 32 controls). Analysis of the cells and protein in the lavage fluids from patients with IPF revealed an inflammatory and eosinophilic response and a significant elevation of IgG in the lungs. With corticosteroid therapy, inflammation diminished but eosinophils remained. Lavage fluid from patients with CHP also had eosinophils and elevated levels of IgG. However, in contrast to IPF, lavage fluid from CHP patients contained IgM, fewer inflammatory cells, and a strikingly increased number (38-74%) of lymphocytes. Identification of lavage lymphocytes in CHP showed that T lymphocytes were significantly elevated and B lymphocytes were decreased compared to peripheral blood. These studies suggest nthat the lung in IPF and CHP may function as a relatively independent immune organ, and that analysis of cells and proteins in broncho-alveolar lavage fluid may be of diagnostic, therapeutic, and investigative value in evaluating patients with fibrotic lung disease.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cantrell E. T., Warr G. A., Busbee D. L., Martin R. R. Induction of aryl hydrocarbon hydroxylase in human pulmonary alveolar macrophages by cigarette smoking. J Clin Invest. 1973 Aug;52(8):1881–1884. doi: 10.1172/JCI107371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele R. P., Altose M. D., Rowlands D. T., Jr Immunocompetent cells from the lower respiratory tract of normal human lungs. J Clin Invest. 1975 Oct;56(4):986–995. doi: 10.1172/JCI108179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. S., Brody A. R., Landis J. N., Graham W. G., Craighead J. E., Green G. M. Quantitation of inflammatory activity in interstitial pneumonitis by bronchofiberscopic pulmonary lavage. Chest. 1976 Feb;69(2 Suppl):265–266. doi: 10.1378/chest.69.2_supplement.265. [DOI] [PubMed] [Google Scholar]
- Frank M. M., Gaither T. The effect of temperature on the reactivity of guinea-pig complement with gamma G and gamma M haemolytic antibodies. Immunology. 1970 Dec;19(6):967–974. [PMC free article] [PubMed] [Google Scholar]
- Gaither T. A., Alling D. W., Frank M. M. A new one-step method for the functional assay of the fourth component (C4) of human and guinea pig complement. J Immunol. 1974 Aug;113(2):574–583. [PubMed] [Google Scholar]
- HANKS J. H., WALLACE J. H. Determination of cell viability. Proc Soc Exp Biol Med. 1958 May;98(1):188–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- Hensley G. T., Fink J. N., Barboriak J. J. Hypersensitivity pneumonitis in the monkey. Arch Pathol. 1974 Jan;97(1):33–38. [PubMed] [Google Scholar]
- Ishizaka K., Newcomb R. W. Presence of gammaE in nasal washings and sputum from asthmatic patients. J Allergy. 1970 Oct;46(4):197–204. doi: 10.1016/0021-8707(70)90023-7. [DOI] [PubMed] [Google Scholar]
- KEIMOWITZ R. I. IMMUNOGLOBULINS IN NORMAL HUMAN TRACHEOBRONCHIAL WASHINGS: A QUALITATIVE AND QUANTITATIVE STUDY. J Lab Clin Med. 1964 Jan;63:54–59. [PubMed] [Google Scholar]
- Kazmierowski J. A., Fauci A. S., Reynolds H. Y. Characterization of lymphocytes in bronchial lavage fluid from monkeys. J Immunol. 1976 Mar;116(3):615–618. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- May J. E., Frank M. M. Complement-mediated tissue damage: contribution of the classical and alternate complement pathways in the Forssman reaction. J Immunol. 1972 Jun;108(6):1517–1525. [PubMed] [Google Scholar]
- McCombs R. P. Diseases due to immunologic reactions in the lungs (first of two parts). N Engl J Med. 1972 Jun 1;286(22):1186–1194. doi: 10.1056/NEJM197206012862205. [DOI] [PubMed] [Google Scholar]
- Moore V. L., Hensley G. T., Fink J. N. An animal model of hypersensitivity pneumonitis in the rabbit. J Clin Invest. 1975 Oct;56(4):937–944. doi: 10.1172/JCI108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R., Fink J. N., Pruzansky J. J., Reed C., Roberts M., Slavin R., Zeiss C. R. Serum immunoglobulin levels in pulmonary allergic aspergillosis and certain other lung diseases, with special reference to immunoglobulin E. Am J Med. 1973 Jan;54(1):16–22. doi: 10.1016/0002-9343(73)90078-8. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Atkinson J. P., Newball H. H., Frank M. M. Receptors for immunoglobulin and complement on human alveolar macrophages. J Immunol. 1975 Jun;114(6):1813–1819. [PubMed] [Google Scholar]
- Reynolds H. Y., Johnson J. S. Structural units of canine serum and secretory immunoglobulin A. Biochemistry. 1971 Jul 20;10(15):2821–2827. doi: 10.1021/bi00791a003. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Schwartz R. H., Bianco A. R., Handwerger B. S., Kahn C. R. Demonstration that monocytes rather than lymphocytes are the insulin-binding cells in preparations of humah peripheral blood mononuclear leukocytes: implications for studies of insulin-resistant states in man. Proc Natl Acad Sci U S A. 1975 Feb;72(2):474–478. doi: 10.1073/pnas.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman R. H., Virchow C., Rowe D. S. IgE levels in external secretions. Int Arch Allergy Appl Immunol. 1973;44(2):242–248. doi: 10.1159/000230933. [DOI] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]



