Abstract
Objectives
To assess whether plasminogen, which is homologous to lipoprotein (a) [Lp(a)], contains pro-inflammatory oxidized phospholipids (OxPL) and whether this has clinical relevance.
Background
OxPL measured on apolipoprotein B-100 (OxPL/apoB), primarily reflecting OxPL on Lp(a), independently predict cardiovascular disease (CVD) events.
Methods
We examined plasminogen from commercially available preparations and plasma from chimpanzees, gorillas, bonobos, cynomolgus monkeys, wild-type, apoE−/−, LDLR−/−, and Lp(a)-transgenic mice, healthy humans and patients with familial hypercholesterolemia, stable CVD and acute myocardial infarction (AMI). Phosphocholine (PC) containing OxPL present on plasminogen were detected directly with liquid chromatography-mass spectrometry (LCMS/MS) and immunologically with monoclonal antibody E06. In vitro clot lysis assays were performed to assess the effect of the OxPL on plasminogen on fibrinolysis.
Results
LC-MS/MS revealed that OxPC fragments were covalently bound to mouse plasminogen. Immunoblot, immunoprecipitation, density gradient ultracentrifugation and ELISA analyses demonstrated that all human and animal plasma samples tested contained OxPL covalently bound to plasminogen. In plasma samples subjected to density gradient fractionation, OxPL were present on plasminogen in non-lipoprotein fractions but on Lp(a) in lipoprotein fractions. Plasma levels of OxPL/apoB and OxPL/apo(a) varied significantly (>25X) among subjects and also strongly correlated with Lp(a) levels. In contrast, OxPL/plasminogen levels were distributed across a relatively narrow range and did not correlate with Lp(a). Enzymatic removal of OxPL from plasminogen resulted in a longer lysis time for fibrin clots (16.25 vs. 11.96 minutes, p=0.007). In serial measurements over 7 months, OxPL/plasminogen levels did not vary in normal subjects or in patients with stable CVD, but increased acutely over the first month and then slowly decreased to baseline in patients following AMI.
Conclusion
These data demonstrate that plasminogen contains covalently bound OxPL that influences fibrinolysis. OxPL on plasminogen represent a second major plasma pool of OxPL, in addition to that present on Lp(a). OxPL present on plasminogen may have pathophysiological implications in AMI and atherothrombosis.
Keywords: plasminogen, oxidized phospholipids, acute coronary syndromes, fibrinolysis, lipoproteins, lipoprotein(a)
INTRODUCTION
Plasminogen plays a key role in the fibrinolytic system and has also been implicated in several other pathophysiological properties including tissue remodeling, angiogenesis, embryogenesis, tumor metastasis, infections, wound healing and leukocyte migration(1). Plasminogen consists of five tandem kringle domains and a protease domain. It is activated to plasmin by physiological activators, such as tissue-type plasminogen activator (tPA), and in turn, plasmin degrades fibrin-rich thrombi through a functional serine protease domain. Kringles are common motifs in coagulation and growth factors and in apolipoprotein (a) [apo(a)].
Lipoprotein (a) [Lp(a)] is composed of apo(a) covalently bound via a single disulfide bond on kringle IV type 9 to apolipoprotein B-100 (apoB) of low density lipoprotein (LDL. Apo(a) is highly homologous to plasminogen and is believed to have evolved from duplication of parts of the plasminogen gene present on the long arm of chromosome 6. The apo(a) gene sits opposite the plasminogen gene on chromosome 6q26–27 and consists of multiple KIV repeats, of which subtype KIV-2 are present in multiple and variable numbers, KV and an inactive protease domain due to a Ser561-Ile562 substitution for Arg561-Val562 present on plasminogen(2). Unlike plasminogen, which is present widely across species, apo(a) appeared late during evolution, approximately 40–60 million years ago, and is present only in humans, non-human primates and old world monkeys, as well as an unrelated version in European hedgehogs. Lp(a) is now generally recognized as a causal, independent, genetic cardiovascular risk factor for coronary artery disease (CAD) and myocardial infarction(3–5).
We made the initial observation that Lp(a) is a preferential lipoprotein carrier of (OxPL) in humans(6–10). We developed an ELISA that quantitates oxidized phospholipids (OxPL) on human apoB particles (OxPL/apoB), which primarily reflects the presence of OxPL on Lp(a). We have demonstrated that OxPL/apoB levels reflect changes in vascular and endothelial function and coronary calcium, identify the presence and progression of carotid and femoral atherosclerosis, and angiographically determined CAD(8,11). Elevated OxPL/apoB levels occur following acute coronary syndromes (ACS)(6) and percutaneous coronary intervention(12) and predict the occurrence of new cardiovascular disease (CVD) events in previously healthy subjects(3,13). Thus, OxPL/apoB appears to reflect the adverse consequences of Lp(a) on cardiovascular outcomes, but are also independently associated with CVD risk above and beyond Lp(a) levels (reviewed in Taleb et al.(14)).
In this study, we examine the hypothesis that plasminogen, due to its high homology to apo(a), may also contain OxPL and assessed whether OxPL levels on plasminogen vary with differences in plasma Lp(a) levels in patients with familial hypercholesterolemia (FH), affect fibrinolysis and vary temporally in patients with stable CAD and following ACS.
METHODS (Detailed description of the Methods is available in the Online Supplement)
Human Subjects
Human plasma were obtained from healthy volunteers, patients with heterozygous FH with highly elevated LDL-C levels but varying Lp(a) levels, and serial samples from patients with stable CAD and acute myocardial infarction (AMI)(6).
Source of Animal Plasma Samples
Plasma samples from bonobos, chimpanzees, gorillas, cynomolgus monkeys, wild type, transgenic Lp(a)(15), apoE−/− and LDL−/− mice were obtained for measurement of OxPL on plasminogen.
Plasminogen and Antibodies
Murine and human plasminogen were purchased commercially. A rabbit polyclonal anti-human plasminogen antibody raised against amino acids 16–105 at the N-terminal end of human plasminogen, a sequence not present in apo(a), was used for immunoblot analysis to avoid cross-reactivity with apo(a). A murine monoclonal anti-human plasminogen antibody not cross-reacting with apo(a) and a polyclonal, biotinylated, guinea pig anti-plasminogen antibody were used as capture and detection antibodies, respectively. Monoclonal antibodies MB47, binding human apolipoprotein B-100, LPA4 binding apo(a) and E06 binding the phosphorylcholine (PC) headgroup of OxPL were previously described (12).
Tandem liquid chromatography, mass spectrometry (LC-MS/MS) analysis of OxPL on mouse plasminogen
LC-MS/MS was utilized to assess the covalent binding of PC containing OxPL on mouse plasminogen using a triple quadruple instrument Thermal TSQ Vantage mass spectrometer coupled with a Waters NanoAcquity autosampler/UPLC system, as previously described(16). Full scan monitoring was carried out to scan a mass range of m/z 350 to 1500. Precursor ion scanning (PIS) monitoring was carried out to identify a product ion of m/z 184, which is characteristic for the PC headgroup.
Immunoblot Analyses
Non-reducing SDS-polyacrylamide gel electrophoresis was carried out using precast gradient gels with 4–12 % polyacrylamide concentrations.
Density Gradient Ultracentrifugation and OxPL on Lipoproteins
Isopycnic density gradient ultracentrifugation was used to fractionate plasma providing 24 fractions plus the non-lipoprotein plasma “bottom” fraction as previously described(10). OxPL on apoB (OxPL/apoB) (14) and OxPL on apo(a)(17) (OxPL/apo(a)) were measured as described.
ELISA to Measure Plasminogen Levels and Oxidized Phospholipids on Plasminogen (OxPL/Plasminogen)
To measure plasminogen levels microtiter well plates were incubated with a mouse monoclonal anti-human plasminogen antibody (Meridian, Inc.) at 5 μg/ml overnight at 4°C, the plates washed, human plasma added (1:32,000 dilution) and plasminogen detected with biotinylated guinea pig anti-human plasminogen antibody using chemiluminescence ELISA.
OxPL/plasminogen was determined in a similar manner except the plasma dilution was 1:400 and biotinylated EO6 was the detection antibody. This assay normalized all wells to the same amount of plasminogen and is therefore independent of plasma plasminogen levels.
Immunoprecipitation
To assess whether OxPL are physically associated with plasminogen, increasing amounts of the murine monoclonal anti-human plasminogen antibody (Meridian), not cross-reacting with apo(a), were added to human plasma to preferentially precipitate plasminogen. Plasminogen, OxPL/plasminogen, Lp(a) and OxPL/apo(a) were then measured in the supernatant.
Phospholipase A2 (PLA2) Treatment of Plasminogen
Plasminogen, free of Lp(a), was purified from fresh-frozen plasma using lysine-Sepharose affinity chromatography and incubated with or without 35 U/ml of PLA2 at 37°C for 90 min and then PLA2 quenched by the addition of phenylmethanesulfonylfluoride. The treated plasminogen was then isolated by lysine-Sepharose and subjected to SDS-PAGE followed by silver staining to verify the absence of degradation. OxPL removal was confirmed by western blot analysis using EO6.
In Vitro Clot Lysis Assay
Assays were performed in a manner similar to that previously published (18). Fibrin clots were formed by the addition of a solution containing 1mg/ml purified fibrinogen and 0.66μM plasminogen (with or without PLA2 treatment) to small, separated aliquots of CaCl2, thrombin, and tPA at final concentrations of 10mM, 6nM and 100pM, respectively. Clot lysis at 37°C was monitored by measurement of turbidity at 405 nm and defined as the time required to reach the midpoint between the maximum and minimum turbidity excursions (tm).
Determination of OxPL on Coagulation Factors with Kringle-like Structures
To determine if kringle domains of other coagulation factors also contain OxPL detectable by EO6, we used commercially available antibodies to specifically capture prothrombin, urokinase and tPA in microtiter well plates from plasma of 6 healthy human individuals and OxPL determined with E06.
RESULTS
Baseline Characteristics of the Human Study Population
The baseline characteristics of the human subjects from whom plasma was derived for the various studies are depicted in Table 1. The characteristics are typical of their underlying diagnoses.
Table 1.
Demographics of Human Study Population
| ACS Study |
||||
|---|---|---|---|---|
| AMI (n=8) | Stable CAD (n=17) | Healthy Subjects (n=18) | FH Patients (n=15) | |
| Age (yrs) | 58±13 | 68±11 | 37±7 | 42±19 |
| Male/female | 8/0 | 13/4 | 11/7 | 9/6 |
| Coronary risk factors | ||||
| Hypertension | 4 (50) | 9 (53) | 1 (5) | 1 (7) |
| Tobacco use | 3 (38) | 2 (12) | 1 (5) | 4 (27) |
| Diabetes mellitus | 2 (25) | 4 (24) | 0 | 0 |
| LDL cholesterol>130 mg/dl | 3 (38) | 2 (12) | 5 (26) | 14 (93) |
| HDL cholesterol>35 mg/dl | 3 (38) | 4 (24) | 0 | 8 (53) |
| Medications | ||||
| Statins | 2 (25) | 9 (53) | 1 (5) | 8 (53) |
| Other lipid modifying agents | 0 | 3 (18) | 0 | 8 (53) |
| Beta-blockers | 2 (25) | 4 (24) | 1 (5) | 6 (40) |
| Calcium channel blockers | 0 | 4 (24) | 0 | 2 (13) |
| ACE/ARB inhibitors | 2 (25) | 3 (18) | 0 | 4 (27) |
| Aspirin | 1 (13) | 17 (100) | 2 (11) | 5 (33) |
| Past medical history | ||||
| MI | 0 | 4 (24) | 0 | 4 (27) |
| CHF | 0 | 1 (6) | 0 | 0 |
| PCI | 0 | 3 (18) | 0 | 1 (7) |
| CABG | 0 | 2 (12) | 0 | 5 (33) |
Data are presented as the mean value ±SD or number (%) of subjects.
ACE = angiotensin-converting enzyme; ARB = adrenergic receptor blocker; CABG = coronary artery bypass graft surgery; CAD = coronary artery disease; CHF = congestive heart failure; HDL = and LDL = high- and low-density lipoprotein, respectively; MI = myocardial infarction; PCI = percutaneous coronary intervention.
Determination of OxPL on mouse plasminogen using LC-MS/MS
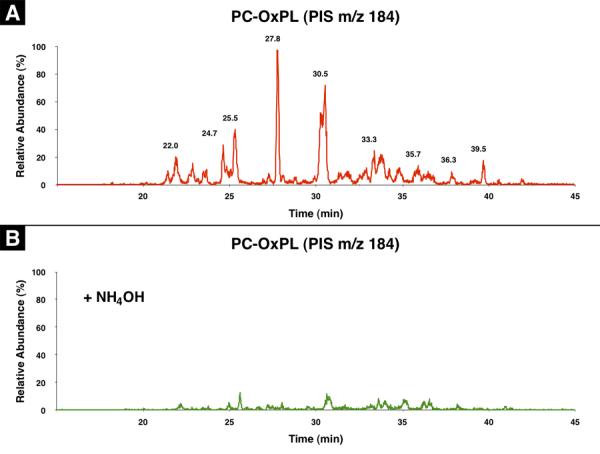
We studied mouse plasminogen [with no possibility of Lp(a) contamination as mice don't have Lp(a)] to determine if covalently bound OxPL containing phosphocholine (OxPC) were present. The presence of OxPC on trypsin digests of plasminogen was assessed by precursor ion scanning (PIS) for m/z184, which is the signature of PC. Since we postulated that the OxPL were covalently bound by Schiff base adducts between oxidized sn-2 side chains of the OxPL and epsilon amino groups of lysine, we also examined for the presence of OxPC on the plasminogen digests before and after saponification with NH4OH.
As shown in Figure 1A, a number of prominent peptide peaks containing OxPC were present in the LC-chromatogram when examined by PIS mass spectrometry, indicating OxPC-modified peptides. More importantly, these peaks disappeared when the protein was first saponified by NH4OH (Figure 1B). Full scan experiments demonstrated the presence of similar patterns of peptides in samples with or without NH4OH pretreatment (data not shown). These results suggest that there are multiple, but limited sites on plasminogen that were covalently modified by OxPC.
Figure 1.
Detection of OxPC on plasminogen by LC-MS/MS. Precursor ion scanning for phosphocholine (m/z 184) was carried out on LC-separated peptides of plasminogen following trypsin digestion without NH4OH treatment (A) and with NH4OH treatment (B). See experimental section for details.
Immunoblot and ELISA Analysis Demonstrating Oxidized Phospholipids on Human and Murine Plasminogen
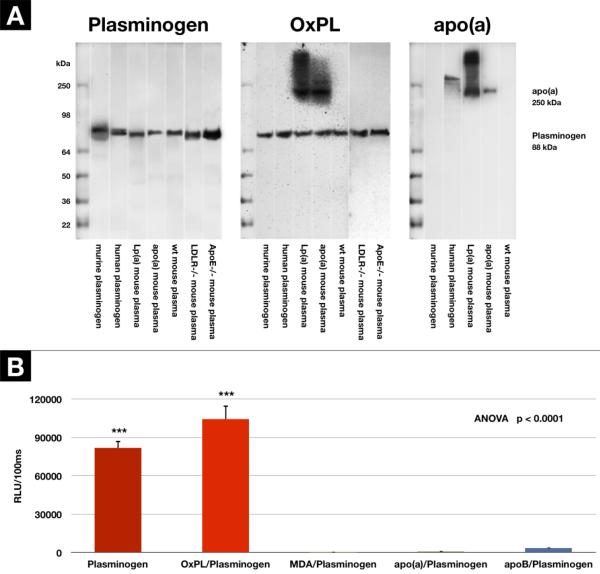
We next examined for the presence of OxPL on plasminogen from a variety of sources by western blotting techniques using monoclonal E06 specific for PC containing OxPL. Polyacrylamide gel electrophoresis of commercially available, lysine sepharose column purified, human and murine plasminogen, as well as freshly procured plasma samples from wild type, LDLR−/, apoE−/− and apo(a) and Lp(a) transgenic mice (all on standard mouse chow) was performed and the gels incubated with 1) biotinylated species-appropriate anti-plasminogen antibodies, 2) monoclonal antibody LPA4 to detect apo(a) and 3) monoclonal antibody EO6 to detect OxPL. Figure 2A (left panel) demonstrates that plasminogen is present in all lanes at the appropriate molecular weight (~88–92 kDa), as expected. Figure 2A (middle panel) demonstrates that OxPL is present in all samples corresponding to the molecular weight of plasminogen and also at the appropriate molecular weight for apo(a) in lanes containing plasma from apo(a) and Lp(a) transgenic mice. There are no other E06 positive bands throughout the gel, suggesting that plasminogen and apo(a)/Lp(a) are the major protein/lipoprotein carriers of OxPL in plasma. Figure 2A (right panel) confirms the presence of apo(a) immunoreactivity, detected by antibody LPA4, in the apo(a) and Lp(a) transgenic mouse plasma. Interestingly, Lp(a) is also present in the commercial preparation of human plasminogen which undoubtedly co-elutes with plasminogen on the lysine-sepharose columns used to purify plasminogen, since they share similar lysine binding sites, particularly on KIV-10. The size of the human Lp(a) is larger than in the Lp(a) transgenic mouse, as the Lp(a)-transgenic mice express a mini apo(a) construct(19). An OxPL band corresponding to the Lp(a) contaminant in the human purified plasminogen is not visible, likely due to much higher (~50×) sensitivity of LPA4 versus E06 on immunoblots, as previously shown(7).
Figure 2.
Presence of PC-containing OxPL on plasminogen and apo(a) demonstrated by Western Blotting and ELISA techniques. A - Immunoblot: Left panel: Specific binding of polyclonal rabbit anti-human plasminogen antibody to plasminogen. Middle panel: OxPL on plasminogen and apo(a) in transgenic mice detected by monoclonal EO6. Right panel: Apo(a) detected by monoclonal LPA4. B - detection of plasminogen, and the presence of OxPL on plasminogen (OxPL/plasminogen), or malondialdehyde associated with plasminogen (MDA/plasminogen), or apo(a) or apoB associated with plasminogen (apo(a) or apoB/plasminogen) by ELISA. Results are mean±SEM and expressed as RLU/100ms. ***p<0.001 compared to the other groups.
Figure 2B displays the plasma plasminogen and OxPL/plasminogen in 6 healthy human subjects using a sandwich ELISA format. It is evident that OxPL are strongly present on plasminogen captured on the microtiter well plate. In contrast, there is no evidence that apo(a) or apoB are present on the captured plasminogen, ruling out non-specific physical interactions of apo(a) or apoB as potential contributions of OxPL on plasminogen. Importantly, like Lp(a), plasminogen is not “oxidized” per se as supported by the observation that murine monoclonal antibody MDA2,(10) which recognizes malondialdehyde (MDA)-lysine epitopes and which are commonly present during generalized lipid peroxidation, does not show immunoreactivity with plasminogen.
Assessment of the Presence of OxPL on Plasminogen Using Immunoprecipitation of Human Plasma
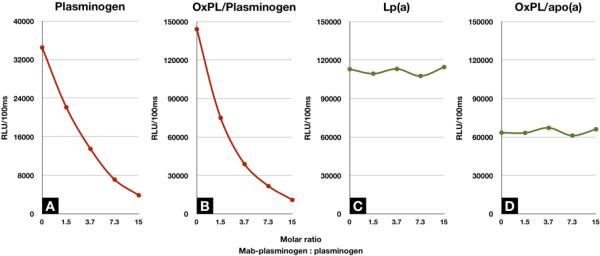
Incubating increasing amounts of a murine monoclonal anti-human plasminogen antibody with human plasma demonstrates that at a molar ratio of ~15:1 (anti-plasminogen antibody:plasminogen) nearly all of the plasminogen was precipitated (Figure 3A). In parallel, a similar decrease in OxPL/plasminogen (Figure 3B) was noted. In contrast, Lp(a) (Figure 3C) and apoB (Figure 3D) remained in the supernatant, suggesting that OxPL were physically associated with plasminogen. In a prior study, we demonstrated that immunoprecipitation of Lp(a) with LPA4 immunoprecipitated the OxPL associated with Lp(a)(10).
Figure 3.
Assessment of the presence of OxPL on plasminogen using immunoprecipitation. This figure displays a representative example of the immunoprecipitation of plasminogen from plasma in a healthy individual with the addition of increasing amounts of anti-plasminogen antibody. The X-axis shows the molar ratio of antibody to plasminogen content added. A: Plasminogen levels remaining in the supernatant following immunoprecipitation. B: OxPL/plasminogen in the supernatant. C: Lp(a) level in supernatant. D: OxPL/apo(a) levels in supernatant.
Coagulation Factors with Kringle-like Structures do not Contain Oxidized Phospholipids
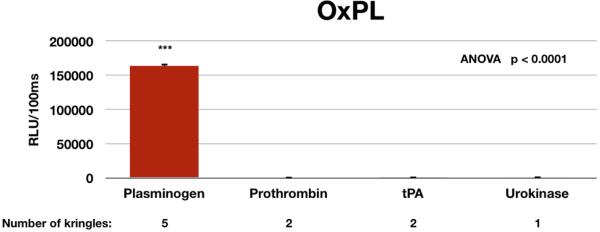
Using specific antibodies to capture plasminogen (5 kringles), prothrombin (factor II) (2 kringles), tissue plasminogen activator (2 kringles), and urokinase (one kringle) on microtiter well plates and adding biotinylated E06, it was demonstrated that OxPL were only present on plasminogen (Figure 4).
Figure 4.
Evaluation of the content of PC-OxPL on coagulation factors with kringle-like structures by double capture ELISA. Plasminogen, prothrombin, tPA and urokinase were captured in microtiter wells by immunocapture from plasma of 6 healthy individuals, and then the content of PC-OxPL determined with monoclonal antibody E06. ***p<0.001 compared to the other groups.
Oxidized Phospholipids on Plasminogen and Lipoproteins Isolated by Density Gradient Ultracentrifugation
Plasmas from 2 patients with an Lp(a) ~90 mg/dl were subjected to density gradient ultracentrifugation. Direct plating of the density fractions on microtiter well plates was used to assess the presence of apo(a) and apoB in each fraction. A set of capture assays was also performed as described above, where specific antibodies for plasminogen, Lp(a) and apoB were used to capture these particles, respectively. Specific antibodies were then used to detect the presence of plasminogen, Lp(a), apoB and OxPL on the captured proteins or lipoproteins. An example from one patient shows that plasminogen was present in the heaviest density fractions and Lp(a) present in the modestly dense fractions (density 1.050–1.090 g/ml, corresponding to fractions 11–15), as expected. These aliquots also directly correspond to the presence of OxPL on plasminogen or Lp(a). ApoB is widely distributed across the density range, but OxPL/apoB is primarily present in the Lp(a) density range, consistent with the fact that these apoB particles are associated with Lp(a) and not LDL (Figure 5). The OxPL/apo(a) and OxPL/apoB and Lp(a) data are also consistent with prior observations from a similar analysis(10).
Figure 5.
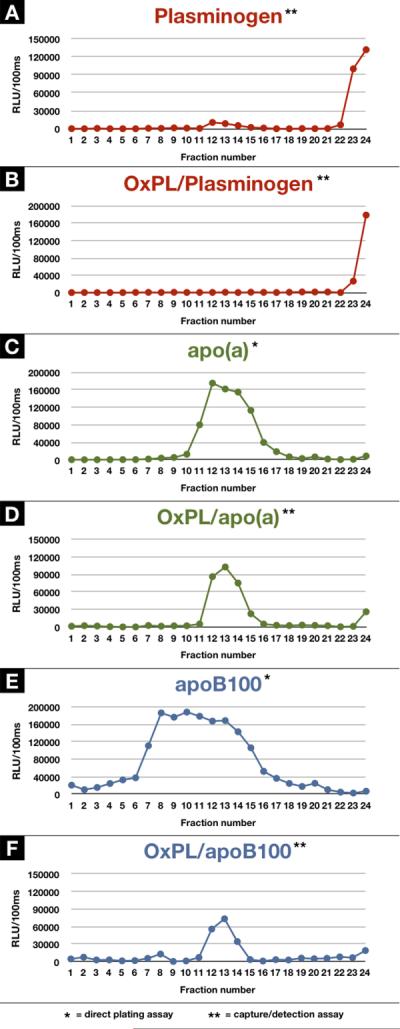
Detection of OxPL on plasminogen using density gradient ultracentrifugation. Density gradient ultracentrifugation from a patient with an Lp(a) plasma level of 90 mg/dl was used to isolate 24 fractions ranging in density 1.000–1.189 g/ml and an aliquot of each fraction was assessed by ELISA for plasminogen (A), OxPL/plasminogen (B), Lp(a) (C), OxPL/Lp(a) (D), apoB (E), and OxPL/apoB (F).
We also examined whether plasminogen levels and OxPL/plasminogen varies among patients with different Lp(a) levels. We analyzed 15 subjects with FH segregated evenly into 3 groups of 5 subjects each, according to low (10±3 mg/dl), intermediate (42±5 mg/dl) and high (103±8 mg/dl) Lp(a) levels (p<0.0001 by ANOVA) (Figure 6). We also assessed the nonlipoprotein containing fraction (“bottom” fraction) for plasminogen and OxPL/plasminogen. It is noted that the plasma OxPL/apo(a) levels (p<0.0001 by ANOVA) track nearly identically with the plasma Lp(a) levels (p<0.0001 by ANOVA) (Figure 6A and 6B), but vary significantly (>25-fold) among groups. However, in contrast, plasminogen levels (p=0.08 by ANOVA) and OxPL/plasminogen (p=0.14 by ANOVA) are not significantly different among the groups (Figure 6C and 6D) and vary minimally (<2-fold). As a confirmation of the presence of plasminogen (p=0.28 by ANOVA) and OxPL/plasminogen (p=0.71 by ANOVA) in nonlipoprotein fractions, we also evaluated the “bottom” density fraction which contains all the nonlipoprotein plasma proteins, and the findings are similar to the plasma data for plasminogen (Figure 6E and 6F).
Figure 6.
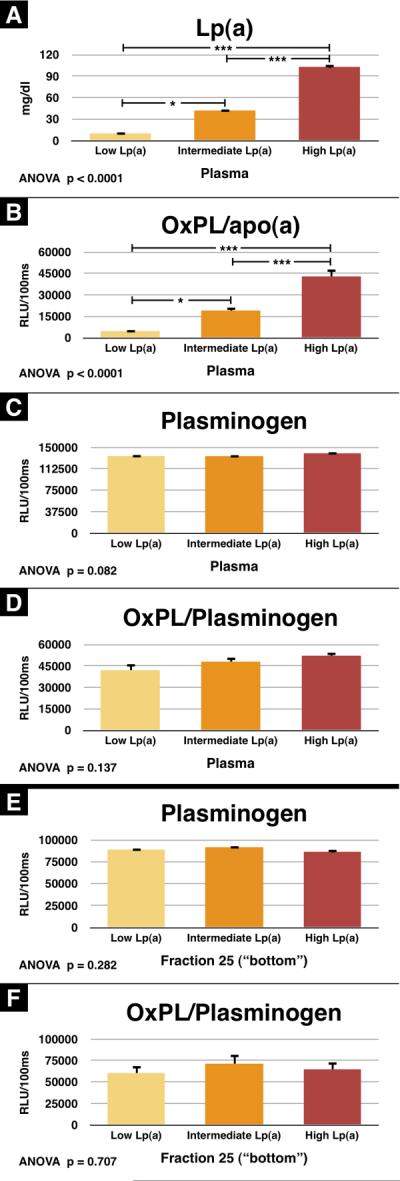
Plasminogen and OxPL/plasminogen from FH patients with different plasma Lp(a) levels. Plasma from 15 FH patients with varying Lp(a) levels was assessed by ELISA for Lp(a) (A), OxPL/apo(a) (B), plasminogen (C), and OxPL/plasminogen (D). Additionally, the bottom fraction was also assessed for plasminogen (E), and OxPL/plasminogen (F). *p<0.05 and ***p<0.001 compared to the other groups as indicated.
Oxidized Phospholipids on Plasminogen of Monkeys and Apes
Plasminogen and OxPL/plasminogen were measured in plasma from 6 cynomolgus monkeys, 14 gorillas, 5 chimpanzees, and 4 bonobos using the same assays used for the human plasma. Oxidized phospholipid was detected on the plasminogen of each species, and the OxPL/plasminogen levels corresponded to the level of plasminogen (Online Figure). Comparison of the relative differences between groups cannot be made as differences in the affinities of the capture and detection antibodies among the species may exist, which were not directly tested due to the difficulty in obtaining purified plasminogen from apes.
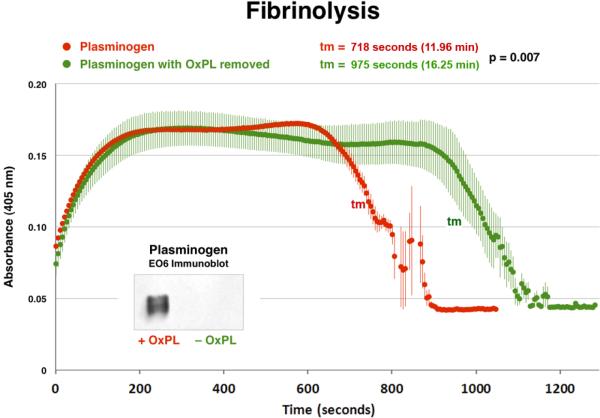
In Vitro Clot Lysis Assay with Native Human Plasminogen
To enzymatically remove the covalently bound OxPL, purified human glu-plasminogen was treated with PLA2 and the OxPL was successfully removed as documented by immunoblotting with E06 (inset, Figure 7). The ability of plasminogen species with and without OxPL to degrade fibrin clots was then tested with a well validated in vitro clot assay(18). This demonstrates that the enzymatic removal of the OxPL component (without degrading the plasminogen protein integrity) results in a 36% longer clot lysis time (975±41.2 vs. 718±6.6 seconds, p=0.007, or 16.25 vs. 11.96 minutes) compared to the intact plasminogen containing OxPL (Figure 7). Clot lysis time is defined as the tm (transition midpoint) that is halfway between the minimum and maximum excursions. This suggests that the presence of OxPL on plasminogen facilitates clot lysis.
Figure 7.
In vitro clot lysis assay assessing the ability of plasminogen to degrade fibrin clots. Native plasminogen containing OxPL and plasminogen with OxPL enzymatically removed (inset) with phospholipase A2 were used. In this system, thrombin-induced clot formation occurs within the first 2 min and is marked by an initial rapid increase in turbidity, as measured by absorbance at 405 nm. Subsequent clot lysis is indicated by a rapid return of the turbidity signal to base-line levels. The parameter tm (transition midpoint) is taken as the standard measure of lysis time and is defined as the time point on the lysis curve that is halfway between the minimum and maximum excursions. The curves represent the mean±SEM of 3 separate experiments with measurement of absorbance at 405 nm every 5 seconds.
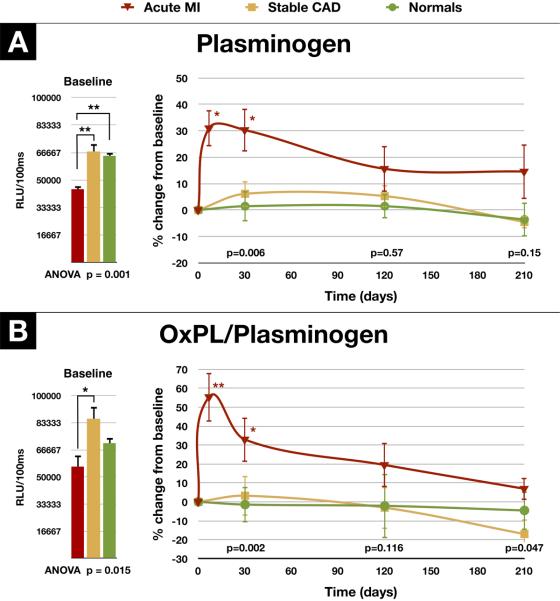
Temporal Trends in Plasminogen, OxPL/Plasminogen in Normal Human Subjects, Patients with Coronary Artery Disease and Acute Coronary Syndromes
To assess changes with time, we measured plasminogen and OxPL/plasminogen levels in serial time points over 7 months in 18 healthy volunteers, 17 patients with stable CAD and 8 patients with AMI, 6 of which had an ST ST-segment elevation myocardial infarction (STEMI) (Figure 8). Interestingly, the baseline levels of plasminogen and OxPL/plasminogen were lower in the AMI patients compared to the healthy subjects and patients with stable CAD (44,432±5,184 RLU, 64,649±9,043 RLU, 67,283±19,821 RLU, respectively, p=0.001 by ANOVA, Figure 8A). These RLU values correspond to plasminogen levels of approximately 15–20 mg/dl based on the standard curve of the plasminogen ELISA. Baseline levels of OxPL/plasminogen levels were also lower in the AMI patients compared to patients with stable CAD but not compared to healthy subjects (56,369±19,290 RLU, 85,809±30,475 RLU, 70,795±15,172, respectively, p=0.015 by ANOVA, Figure 8B).
Figure 8.
Change in plasminogen and OxPL/plasminogen in normal subjects, patients with stable coronary artery disease and acute myocardial infarction. Panel A shows the baseline levels and changes in plasminogen levels over a 7 month period in patients following AMI (n=8), in patients with stable CAD (n=17) and in healthy subjects (n=18). Panel B shows the baseline levels and changes in OxPL/plasminogen over the same time period. The p-values at the bottom of the figures represent the discharge (average of 4 days for the AMI group) and 30, 120, and 210-day differences between groups at each time point. *p<0.05 and **p<0.01 represent Bonferroni post test for changes within groups over time.
Evaluating the data as a mean percent change over time across each group by ANOVA, the plasminogen levels were significantly elevated in the AMI group at discharge (p=0.01) and after 30 days (p=0.01), but not after 120 days and 7 months (Figure 8B). The OxPL/plasminogen levels were also elevated at discharge (p=0.01) and after 30 days (p=0.05), but not after 120 days and 7 months (Figure 8B). In contrast, there were no significant changes in plasminogen levels and OxPL/plasminogen in normal individuals (p=0.86 and p=0.98 by ANOVA) and patients with stable CAD (p=0.46 and p=0.31 by ANOVA) over time. For comparison between groups, significant differences were noted at the 30 day timepoint for both plasminogen and OxPL/plasminogen but not at the other timepoints. Plasminogen and OxPL/plasminogen levels did not correlate with Lp(a), OxPL/apoB or OxPL/apo(a) levels (data not shown).
DISCUSSION
This study demonstrates that plasminogen is a major carrier of OxPL in plasma of humans and animals and appears to be important in facilitating fibrinolysis. OxPL on plasminogen are distinct from the OxPL present on Lp(a) and represent the second major pool of OxPL in plasma. Unlike OxPL/apoB and OxPL/apo(a) levels, which vary widely and which were previously shown to correlate with plasma Lp(a) levels(3,9), OxPL/plasminogen levels are distributed in a very narrow range and do not change over time among healthy subjects and patients with stable CAD. However, they rise acutely in patients following AMI. These findings may link atherogenic and thrombotic processes in defining the presence of OxPL as a unifying pathophysiological link among Lp(a) and plasminogen.
Glu-plasminogen is the zymogen that is converted to the active protease “plasmin” by cleavage of Arg561-Val562 resulting in an N-terminal heavy chain of 561-amino acids and a disulfide-linked C-terminal light chain of 230-amino acids. Plasmin can also activate gluplasminogen to lys-plasminogen by removing the first N-terminal 77 amino acids in a positive feedback reaction. The catalytic triad of plasmin consists of His603, Asp646, and Ser741. Kringles I and IV participate in binding to lysine residues present on the surface of fibrin or cell membranes. We now present evidence in a variety of animal models, healthy humans and patients with CAD and ACS that plasminogen is a major carrier of a distinct pool of OxPL within the non-lipoprotein fraction of plasma that is not associated with Lp(a). The LC-MS/MS results demonstrate that the OxPC covalently modify plasminogen. The precursor ion scanning experiments were carried out on peptides using a collision energy of 35V that are indicative of the covalent nature of these modifications. One would expect that non-covalent association of PC species with plasminogen would not survive the stringent condition of denaturing, reduction, and digestion. Furthermore, disruption of non-covalent interaction in a collision induced dissociation experiment requires much less energy. Finally, the demonstration that saponification removed the phosphocholine from the peptides is consistent with the postulated Schiff base formation between lysines of the protein and the reactive oxidized moieties present on the sn-2 side chains of the OxPL(20).
Although phospholipid surfaces are needed to activate clotting cascades, the role of OxPL in these pathways is not well defined. The few studies published in this area are not entirely consistent, but on the whole suggest free OxPL species promote a pro-coagulant shift on the endothelium and mediate blood clotting(21). In addition, OxPL in concert with hyperlipidemia induce platelet aggregation through a CD36 scavenger receptor pathway in platelets(22). The current study suggests for the first time that OxPL on plasminogen are also important in facilitating fibrinolysis, as their removal results in a longer clot lysis time. This removal of OxPL may result in a decreased rate of plasminogen activation or a decreased enzymatic activity of plasmin. It may be postulated that the presence of OxPL on plasminogen may facilitate activation of plasminogen to plasmin by tPA and other plasminogen activators. Furthermore, the OxPL on plasminogen may bind to scavenger receptors on cells that are recruited at sites of inflammation, atherosclerosis and thrombosis. It remains to be defined what percent of circulating plasminogen contains covalently bound OxPL and what their relative contributions to fibrinolysis are. In contrast to the above, Lp(a) has been shown to inhibit fibrinolysis in vitro(18,23,24), but the specific role of the OxPL component on Lp(a) on fibrinolysis has not been investigated. The overall role of OxPL on plasminogen and Lp(a) in mediating all aspects of fibrinolysis awaits further dedicated mechanistic studies.
In vitro tissue culture studies have shown that the gene expression of plasminogen can increase significantly when cells are exposed to inflammatory mediators, such as IL-6(25). In the current study, we demonstrate for the first time in serial time points that both plasminogen and OxPL/plasminogen levels were significantly lower at baseline in patients with AMI compared to stable CAD and normal subjects, but increase acutely following the pro-inflammatory milieu of AMI in subjects treated with PCI. Although this AMI study is small and hypothesis generating, it does imply that OxPL/plasminogen may vary before and following plaque rupture and thrombosis and that they may play a role in either thrombosis and fibrinolysis, the extent of reperfusion and perhaps long term clinical outcomes. An acute increase in plasminogen levels post AMI was previously documented, although the clinical implications remain undefined(26). Interestingly, a similar pattern of changes in Lp(a), OxPL/apoB and autoantibodies to OxLDL were previously noted in the same subjects, as well as in PCI patients in another cohort(6,12). Since plasminogen (like apoB) is usually in excess to apo(a), except in cases of very high Lp(a) levels, it suggests that plasminogen may represent a larger carrier of OxPL than Lp(a). We are currently evaluating the changes in OxPL/plasminogen and plasminogen in large ACS studies and assessing their predictive value.
Interestingly, animal and human studies have suggested, but not proven definitively, that elements of the fibrinolytic system may have pro-atherogenic properties(27). In fact, experimental studies have suggested that plasminogen mediates pro-atherogenic effects in mouse models(28,29). Although these data need confirmation, one may postulate that some of these effects may be mediated through OxPL and CD36 macrophage scavenger receptor pathway(30,31). The presence of OxPL on plasminogen may also activate smooth muscle cells, enhance endothelial cells growth or induce release of proinflammatory cytokines that theoretically may be beneficial in injured tissues, but detrimental in atherosclerotic lesions. The identification of specific lysine receptors on a number of cell surfaces and bacteria have implicated plasminogen in additional functions such as facilitating tissue remodeling, enhancing wound healing, mediating angiogenesis and embryogenesis, and inhibiting infection, tumor growth and metastasis(1). The presence of OxPL on plasminogen may mediate clearance of such pathogens by recruiting additional arcs of the innate immune system, such as natural antibodies, scavenger receptors, C-reactive protein, and potentially Lp(a), which has also been postulated to be involved in wound healing(1,32). Plasminogen deficient patients(33) and murine models of plasminogen deficiency support many of these functions of plasminogen manifested by diffuse fibrin deposition leading to multiple organ failure(34,35).
Substantial experimental, clinical and genetic evidence have established Lp(a) as an independent risk factor for premature CAD, death, MI, stroke and peripheral arterial disease, although the mechanisms underlying its pro-atherogenic potential are not fully established(3–5). Lp(a) has also been shown to inhibit the fibrinolytic properties of plasminogen in vitro through several mechanisms, including inhibiting tPA mediated activation of glu-plasminogen(18,24), inhibition of plasminogen and tPA lysine-dependent binding to fibrin surfaces(36), and inhibiting the action of plasmin in converting glu-plasminogen to the activated form lys-plasminogen(23). In a large series of clinical and experimental studies(9), we have established that a key component of the atherogenicity of Lp(a) and its value in predicting new cardiovascular events(3,13) may be its unique property, among lipoproteins, to preferentially bind and transport pro-inflammatory OxPL. Measuring OxPL on apoB particles (OxPL/apoB), which primarily reflects OxPL on Lp(a), appears to uniquely reflect the biological activity and pro-atherogenic potential of Lp(a), particularly of the most atherogenic small isoforms of apo(a) associated with high Lp(a) levels(14). As opposed to plasminogen, Lp(a) contains not only covalently bound OxPL, but also OxPL present in its lipid phase(10).
It appears that only Lp(a) and plasminogen contain significant amounts of OxPL in plasma proteins, as no other immunoreactive bands can be seen in immunoblots of all species tested. There was no evidence that plasminogen was attached to OxPL-containing Lp(a) or apoB particles circulating in plasma. Kringles of the plasminogen-prothrombin gene family share conformational epitopes with each other and with apo(a), but plasminogen and Lp(a) are the only kringle containing structures containing OxPL, although all coagulation and growth factors were not tested in this study. This suggests that it is not the kringle structures per se that mediate OxPL binding, but other as yet unidentified motif(s) common to both apo(a) and plasminogen. Edelstein et al.(37) recently reported that commercial and cell culture sources of human plasminogen contained covalently bound OxPL as detected by several techniques, including immunoreactivity of the antibody T15(37), which our group initially demonstrated to be identical in the variable region to the IgM E06(38), inorganic phosphate analysis, and presence of lyso-PC from plasminogen following treatment with lipoprotein-associated phospholipase A2. Their study did not evaluate non-human sources of plasminogen or evaluate its role in lipoprotein disorders and cardiovascular diseases, nor did it differentiate Lp(a)/apo(a) versus plasminogen pools in plasma. Studies are underway to define the specific binding sites and amino acids on plasminogen and Lp(a) that bind OxPL. This would also allow a determination of the affinity and relative proportion of plasminogen molecules that carry OxPL.
Conclusion
Our findings demonstrate that plasminogen contains covalently bound OxPL and that plasminogen represents a second major pool of OxPL in human plasma in addition to Lp(a). OxPL on plasminogen seem to facilitate fibrinolysis and that patients with ACS develop elevated levels of both plasminogen and OxPL/plasminogen. The presence of OxPL on plasminogen may have pathophysiological implications in atherothrombosis and clinical events and await future basic and clinical investigations.
Supplementary Material
Acknowledgments
Funding Sources This study was funded by a grant from the Fondation Leducq (ST, MJC, JLW), the Swiss National Science Foundation (G.L.), NIH HL0888093, HL086559 (JLW, ST) and ES013125 (HY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures Drs. Tsimikas and Witztum are inventors of patents owned by the University of California for the clinical use of oxidation-specific antibodies.
References
- 1.Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93:647–54. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- 2.McLean JW, Tomlinson JE, Kuang WJ, et al. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature. 1987;330:132–7. doi: 10.1038/330132a0. [DOI] [PubMed] [Google Scholar]
- 3.Tsimikas S, Mallat Z, Talmud PJ, et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56:946–55. doi: 10.1016/j.jacc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 4.The Emerging Risk Factors C. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 6.Tsimikas S, Bergmark C, Beyer RW, et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41:360–70. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 7.Edelstein C, Pfaffinger D, Hinman J, et al. Lysine-phosphatidylcholine adducts in kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein(a) J Biol Chem. 2003;278:52841–7. doi: 10.1074/jbc.M310425200. [DOI] [PubMed] [Google Scholar]
- 8.Tsimikas S, Brilakis ES, Miller ER, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 9.Tsimikas S, Witztum JL. The role of oxidized phospholipids in mediating lipoprotein(a) atherogenicity. Curr Opin Lipidol. 2008;19:369–77. doi: 10.1097/MOL.0b013e328308b622. [DOI] [PubMed] [Google Scholar]
- 10.Bergmark C, Dewan A, Orsoni A, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. Journal of lipid research. 2008;49:2230–9. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Tsimikas S, Kiechl S, Willeit J, et al. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J Am Coll Cardiol. 2006;47:2219–28. doi: 10.1016/j.jacc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Tsimikas S, Lau HK, Han KR, et al. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164–70. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- 13.Kiechl S, Willeit J, Mayr M, et al. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler Thromb Vasc Biol. 2007;27:1788–95. doi: 10.1161/ATVBAHA.107.145805. [DOI] [PubMed] [Google Scholar]
- 14.Taleb A, Witztum JL, Tsimikas S. Oxidized Phospholipids on Apolipoprotein B-100 (OxPL/apoB) Containing Lipoproteins: A Biomarker Predicting Cardiovascular Disease and Cardiovascular Events. Biomarkers Med. 2011;5:673–694. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merki E, Graham MJ, Mullick AE, et al. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation. 2008;118:743–53. doi: 10.1161/CIRCULATIONAHA.108.786822. [DOI] [PubMed] [Google Scholar]
- 16.Szapacs ME, Kim HY, Porter NA, Liebler DC. Identification of proteins adducted by lipid peroxidation products in plasma and modifications of apolipoprotein A1 with a novel biotinylated phospholipid probe. J Proteome Res. 2008;7:4237–46. doi: 10.1021/pr8001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faghihnia N, Tsimikas S, Miller ER, Witztum JL, Krauss RM. Changes in lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J Lipid Res. 2010;51:3324–30. doi: 10.1194/jlr.M005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sangrar W, Bajzar L, Nesheim ME, Koschinsky ML. Antifibrinolytic effect of recombinant apolipoprotein(a) in vitro is primarily due to attenuation of tPA-mediated Glu-plasminogen activation. Biochemistry. 1995;34:5151–7. doi: 10.1021/bi00015a028. [DOI] [PubMed] [Google Scholar]
- 19.Schneider M, Witztum JL, Young SG, et al. High-level lipoprotein [a] expression in transgenic mice: evidence for oxidized phospholipids in lipoprotein [a] but not in low density lipoproteins. J Lipid Res. 2005;46:769–78. doi: 10.1194/jlr.M400467-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Gillotte KL, Horkko S, Witztum JL, Steinberg D. Oxidized phospholipids, linked to apolipoprotein B of oxidized LDL, are ligands for macrophage scavenger receptors. Journal of lipid research. 2000;41:824–33. [PubMed] [Google Scholar]
- 21.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–59. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nature Medicine. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feric NT, Boffa MB, Johnston SM, Koschinsky ML. Apolipoprotein(a) inhibits the conversion of Glu-plasminogen to Lys-plasminogen: a novel mechanism for lipoprotein(a)-mediated inhibition of plasminogen activation. J Thromb Haemost. 2008;6:2113–20. doi: 10.1111/j.1538-7836.2008.03183.x. [DOI] [PubMed] [Google Scholar]
- 24.Hancock MA, Boffa MB, Marcovina SM, Nesheim ME, Koschinsky ML. Inhibition of plasminogen activation by lipoprotein(a): critical domains in apolipoprotein(a) and mechanism of inhibition on fibrin and degraded fibrin surfaces. J Biol Chem. 2003;278:23260–9. doi: 10.1074/jbc.M302780200. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins GR, Seiffert D, Parmer RJ, Miles LA. Regulation of Plasminogen Gene Expression by Interleukin-6. Blood. 1997;89:2394–2403. [PubMed] [Google Scholar]
- 26.Meltzer ME, Doggen CJM, de Groot PG, Rosendaal FR, Lisman T. Plasma levels of fibrinolytic proteins and the risk of myocardial infarction in men. Blood. 2010;116:529–536. doi: 10.1182/blood-2010-01-263103. [DOI] [PubMed] [Google Scholar]
- 27.Folsom AR, Aleksic N, Park E, Salomaa V, Juneja H, Wu KK. Prospective Study of Fibrinolytic Factors and Incident Coronary Heart Disease : The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 2001;21:611–617. doi: 10.1161/01.atv.21.4.611. [DOI] [PubMed] [Google Scholar]
- 28.Kremen M, Krishnan R, Emery I, et al. Plasminogen mediates the atherogenic effects of macrophage-expressed urokinase and accelerates atherosclerosis in apoE-knockout mice. PNAS. 2008;105:17109–17114. doi: 10.1073/pnas.0808650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Q, Danton MJS, Witte DP, et al. Plasminogen deficiency accelerates vessel wall disease in mice predisposed to□atherosclerosis. PNAS. 1997;94:10335–10340. doi: 10.1073/pnas.94.19.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boullier A, Friedman P, Harkewicz R, et al. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46:969–76. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg ME, Li XM, Gugiu BG, et al. The Lipid Whisker Model of the Structure of Oxidized Cell Membranes. J Biol Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 32.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–48. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tefs K, Gueorguieva M, Klammt J, et al. Molecular and clinical spectrum of type I plasminogen deficiency: a series of 50 patients. Blood. 2006;108:3021–3026. doi: 10.1182/blood-2006-04-017350. [DOI] [PubMed] [Google Scholar]
- 34.Iwaki T, Malinverno C, Smith D, et al. The generation and characterization of mice expressing a plasmin-inactivating active site mutation. J Thromb Haemost. 2010 doi: 10.1111/j.1538-7836.2010.03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ploplis VA, Carmeliet P, Vazirzadeh S, et al. Effects of disruption of the plasminogen gene on thrombosis, growth, and health in mice. Circulation. 1995;92:2585–93. doi: 10.1161/01.cir.92.9.2585. [DOI] [PubMed] [Google Scholar]
- 36.Rouy D, Grailhe P, Nigon F, Chapman J, Angles-Cano E. Lipoprotein(a) impairs generation of plasmin by fibrin-bound tissue-type plasminogen activator. In vitro studies in a plasma milieu. Arterioscler Thromb. 1991;11:629–38. doi: 10.1161/01.atv.11.3.629. [DOI] [PubMed] [Google Scholar]
- 37.Edelstein C, Pfaffinger D, Yang M, Hill JS, Scanu AM. Naturally occurring human plasminogen, like genetically related apolipoprotein(a), contains oxidized phosphatidylcholine adducts. Biochim Biophys Acta. 2010;1801:738–45. doi: 10.1016/j.bbalip.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw PX, Horkko S, Chang MK, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–40. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.