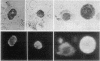
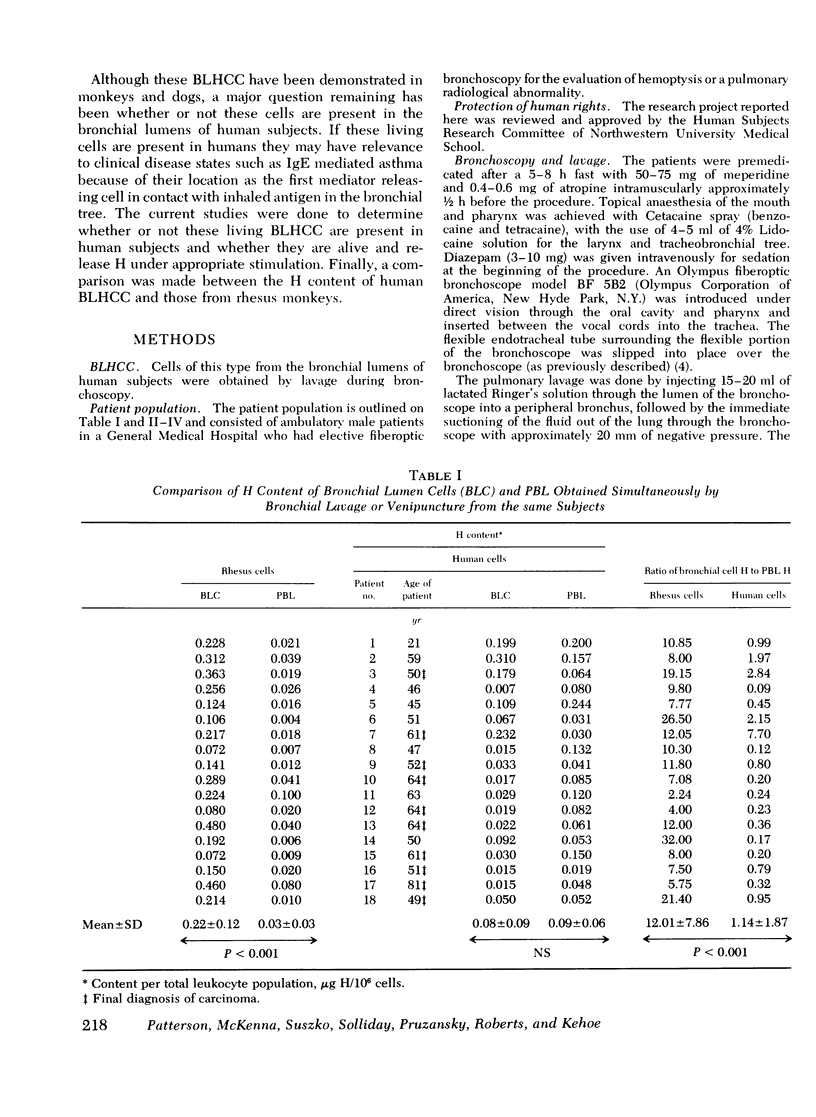
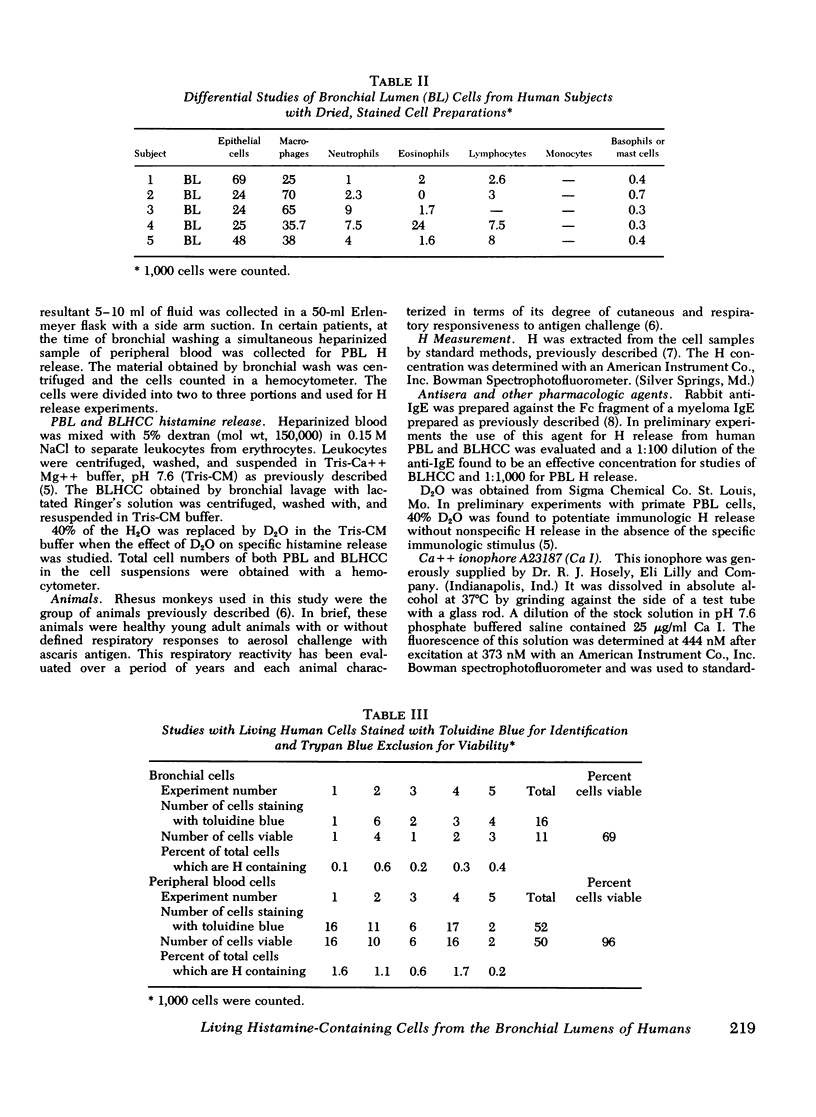
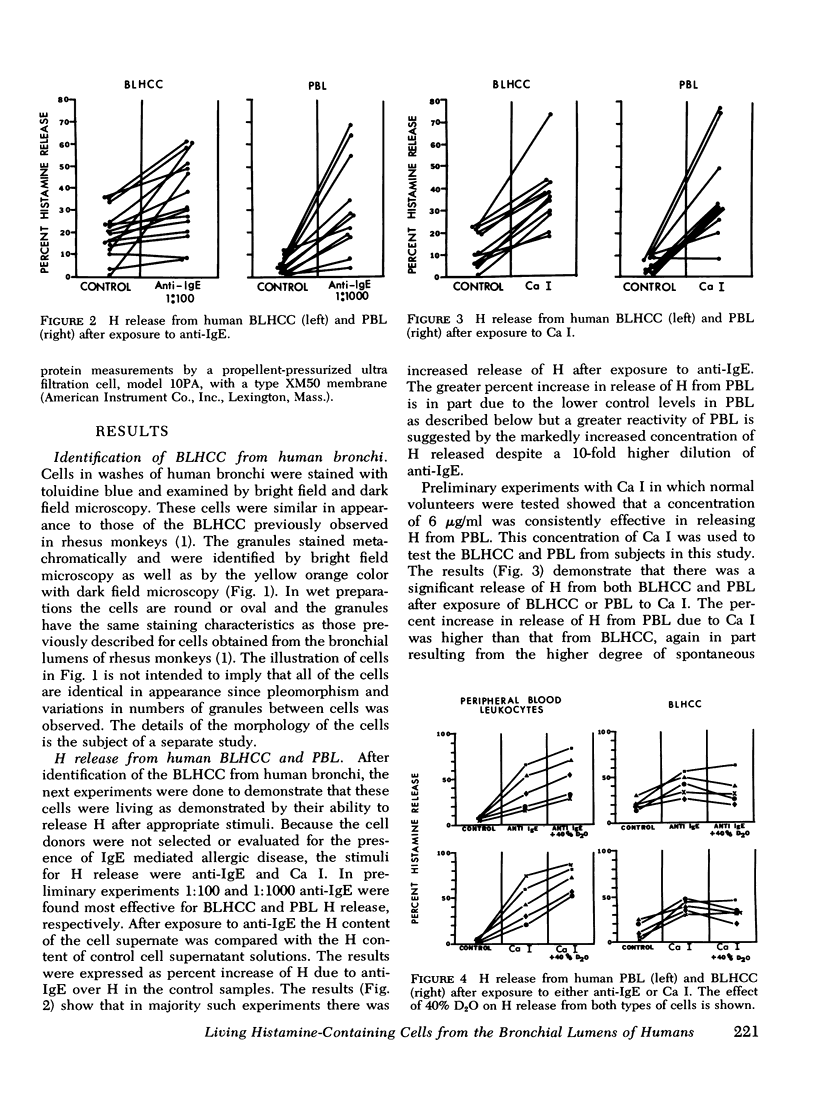
Abstract
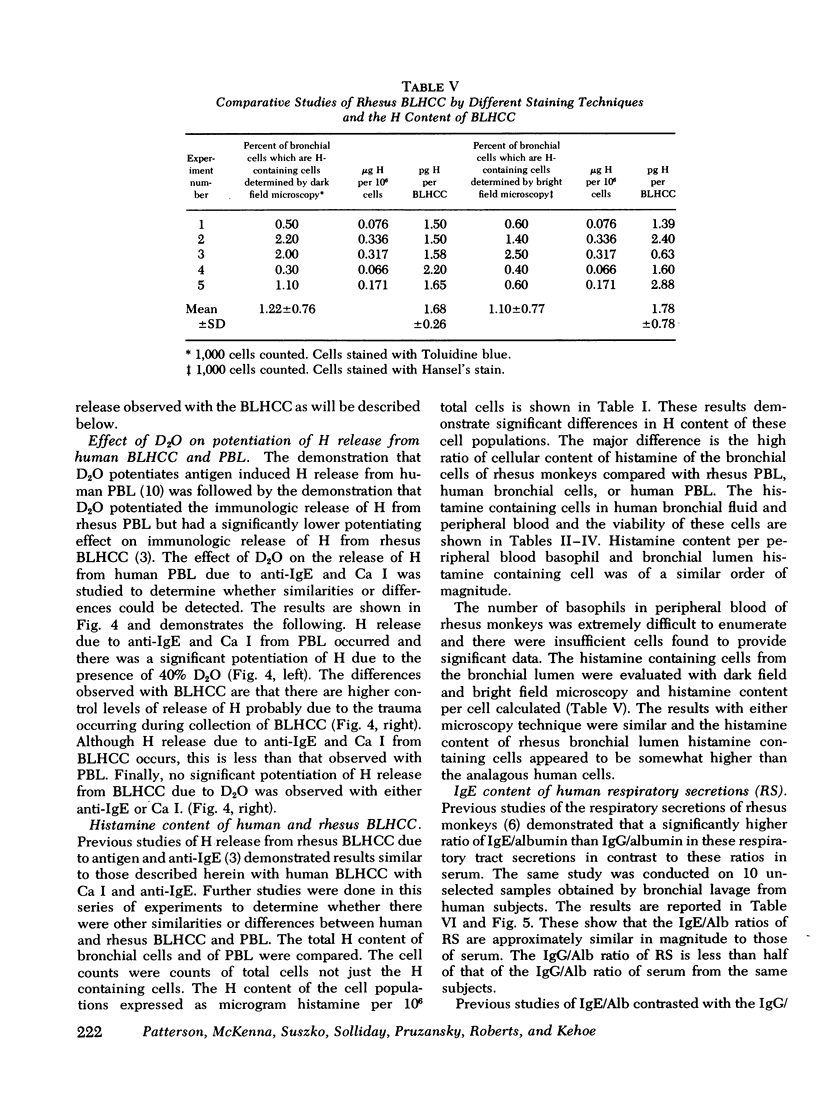
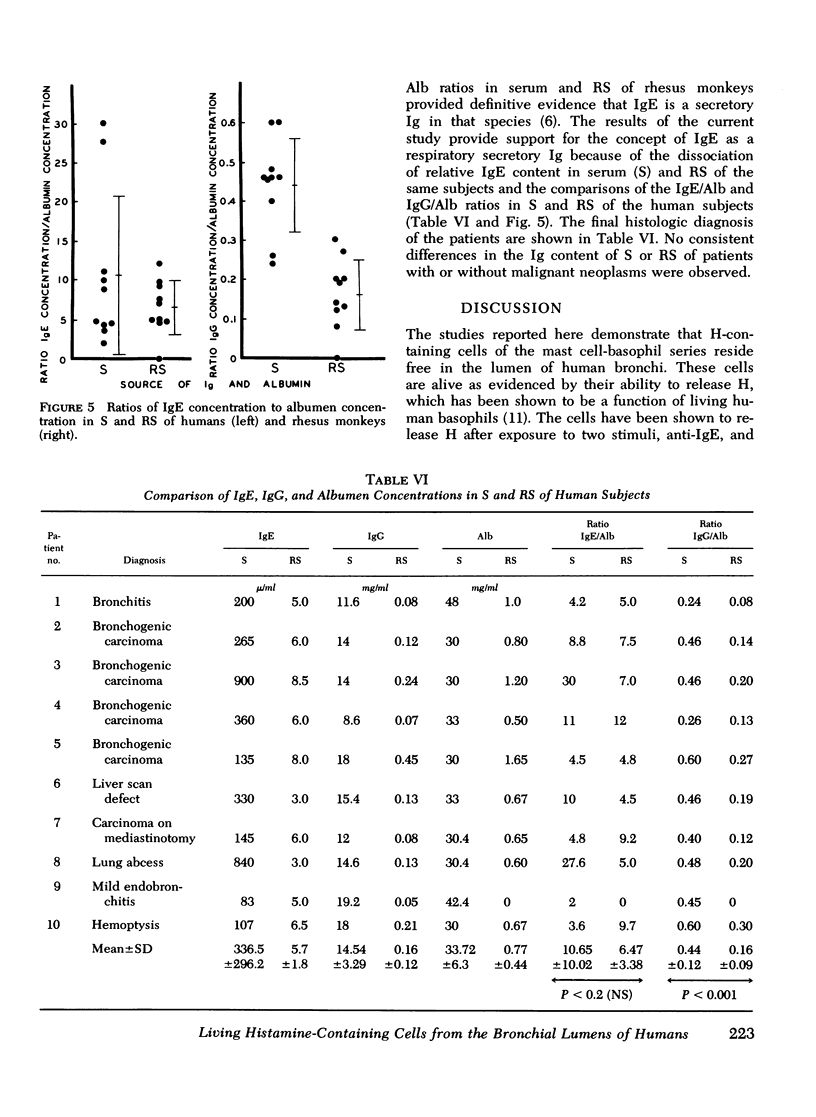
Cell populations obtained by bronchial lavage from human subjects were examined for the presence of cells related to the mast cell-basophil series. Such bronchial lumen histamine-containing cells (BLHCC) were identified. The BLHCC stained with toluidine blue may be identified by bright field or dark field microscopy. The BLHCC are alive as evidenced by ability to release histamine (H) after exposure to anti-IgE or calcium ionophore. Although H release from peripheral blood leukocytes by these two agents is potentiated by the presence of D2O, H release from BLHCC of the same subjects by anti-IgE or calcium ionophore was not potentiated by D2O. In studies comparing bronchial cell populations of humans and rhesus monkeys with peripheral blood leukocyte populations of the same subjects, the histamine content of the bronchial cell population was much higher in rhesus monkeys. IgE/Alb ratios of respiratory secretions and serum of the same human subjects were of the same order of magnitude in contrast to previous comparisons done on these fluids in rhesus monkeys.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Gillespie E., Lichtenstein L. M. Histamine release from human leukocytes: studies with deuterium oxide, colchicine, and cytochalasin B. J Clin Invest. 1972 Nov;51(11):2941–2947. doi: 10.1172/JCI107118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G. J., Averbeck A. K., Swedlund H. A. Measurement of IgE in normal and allergic serum by radioimmunoassay. J Lab Clin Med. 1971 Apr;77(4):690–698. [PubMed] [Google Scholar]
- Johnson A. R., Hugli T. E., Müller-Eberhard H. J. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975 Jun;28(6):1067–1080. [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M. The mechanism of basophil histamine release induced by antigen and by the calcium ionophore A23187. J Immunol. 1975 Jun;114(6):1692–1699. [PubMed] [Google Scholar]
- Osler A. G., Lichtenstein L. M., Levy D. A. In vitro studies of human reaginic allergy. Adv Immunol. 1968;8:183–231. doi: 10.1016/s0065-2776(08)60467-8. [DOI] [PubMed] [Google Scholar]
- Patterson R., Chakrin L. W., Suszko I. M., Mengel J., Wardell J. R., Jr IgE-mediated histamine and SRS-A release from respiratory cells and peripheral blood leukocytes of rhesus monkeys. J Lab Clin Med. 1976 Jun;87(6):1016–1024. [PubMed] [Google Scholar]
- Patterson R., Harris K. E., Suszko I. M., Roberts M. Reagin-mediated asthma in rhesus monkeys and relation to bronchial cell histamine release and airway reactivity to carbocholine. J Clin Invest. 1976 Mar;57(3):586–593. doi: 10.1172/JCI108314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R., Kelly J. F. Animal models of the asthmatic state. Annu Rev Med. 1974;25(0):53–68. doi: 10.1146/annurev.me.25.020174.000413. [DOI] [PubMed] [Google Scholar]
- Patterson R., Suszko I. M., Harris K. E. Potentiation of IgE-mediated cutaneous reactivity and blood leucocyte histamine release by deuterium oxide. Clin Exp Immunol. 1975 Feb;19(2):335–342. [PMC free article] [PubMed] [Google Scholar]
- Patterson R., Talbot C. H., Roberts M. Reverse passive respiratory reactions due to anti-IgE in rhesus monkeys. Clin Exp Immunol. 1972 Feb;10(2):267–274. [PMC free article] [PubMed] [Google Scholar]
- Patterson R., Tomita Y., Oh S. H., Suszko I. M., Pruzansky J. J. Respiratory mast cells and basophiloid cells. I. Evidence that they are secreted into the bronchial lumen, morphology, degranulation and histamine release. Clin Exp Immunol. 1974 Feb;16(2):223–234. [PMC free article] [PubMed] [Google Scholar]
- Pruzansky J. J., Patterson R. Histamine release from leukocytes of hypersensitive individuals. I. Use of several antigens. J Allergy. 1966 Nov;38(5):315–320. doi: 10.1016/0021-8707(66)90028-1. [DOI] [PubMed] [Google Scholar]
- Solomon D. A., Solliday N. H., Gracey D. R. Cytology in fiberoptic bronchoscopy. Comparison of bronchial brushing, washing and post-bronchoscopy sputum. Chest. 1974 Jun;65(6):616–619. doi: 10.1378/chest.65.6.616. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Patterson R., Suszko I. M. Respiratory mast cells and basophiloid cells. II. Effect of pharmocologic agents on 3'5'-adenosine monophosphate content and on antigen-induced histamine release. Int Arch Allergy Appl Immunol. 1974;47(2):261–272. [PubMed] [Google Scholar]