Abstract
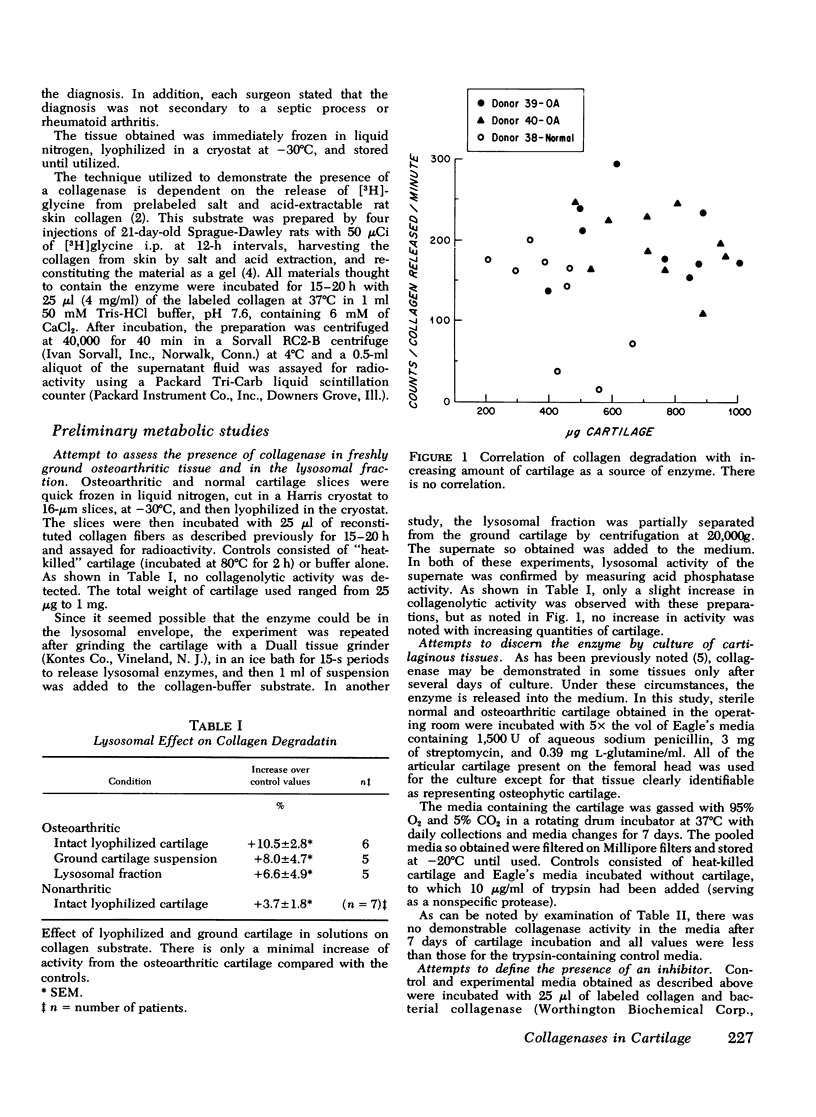
In advanced osteoarthritis, all of the cartilaginous components are lost from the joint surface. Although mechanisms exist for proteoglycan degradation, there is not known to be any system for removal of the collagen. This study suggests that the loss of the collagen components may be a function of articular cartilage collagenase. The enzyme in normal human cartilage is bound to an inhibitor and appears to be present in very small amounts. Attempts to demonstrate collagenase activity in ground human articular cartilage or in its lysosomal fraction were unsuccessful. 7-Day cartilage tissue cultures also failed to demonstrate the presence of the enzyme; but the same culture fluid, incubated with trypsin, showed significant degradation of collagen, suggesting that trypsin destroyed the inhibitor. 7-Day culture fluids were then chromatographed on a heparin-charged Sepharose 4B affinity column that had been activated with cyanogen bromide. This removed the inhibitor, and the chromatographed fluid from osteoarthritic cartilage released 42% of the incorporated counts of the collagen substrate, whereas normal cartilage released 10.1% and a trypsin control, 6.4%. Electrophoresis of the degradation products of the enzyme-collagen complex incubated at 37 degrees C revealed breakdown was complete to small dialyzable fragments, while at 25 degrees C larger fragments were split off.
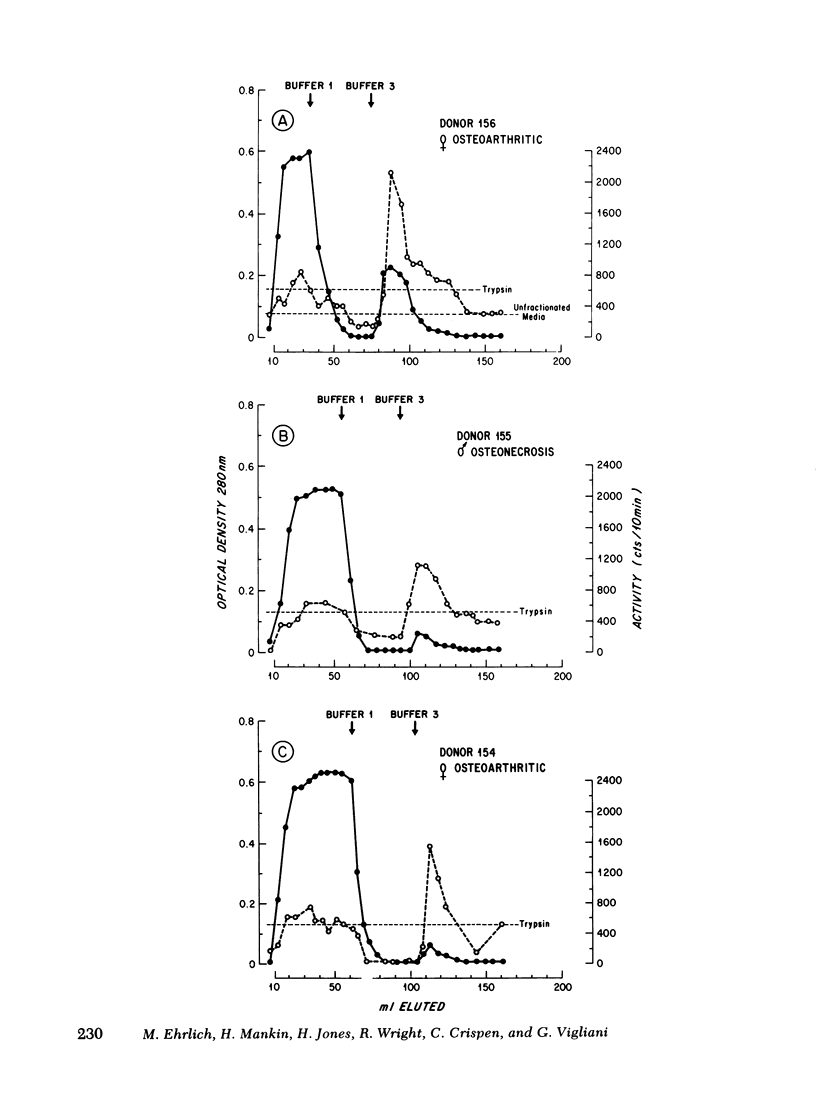
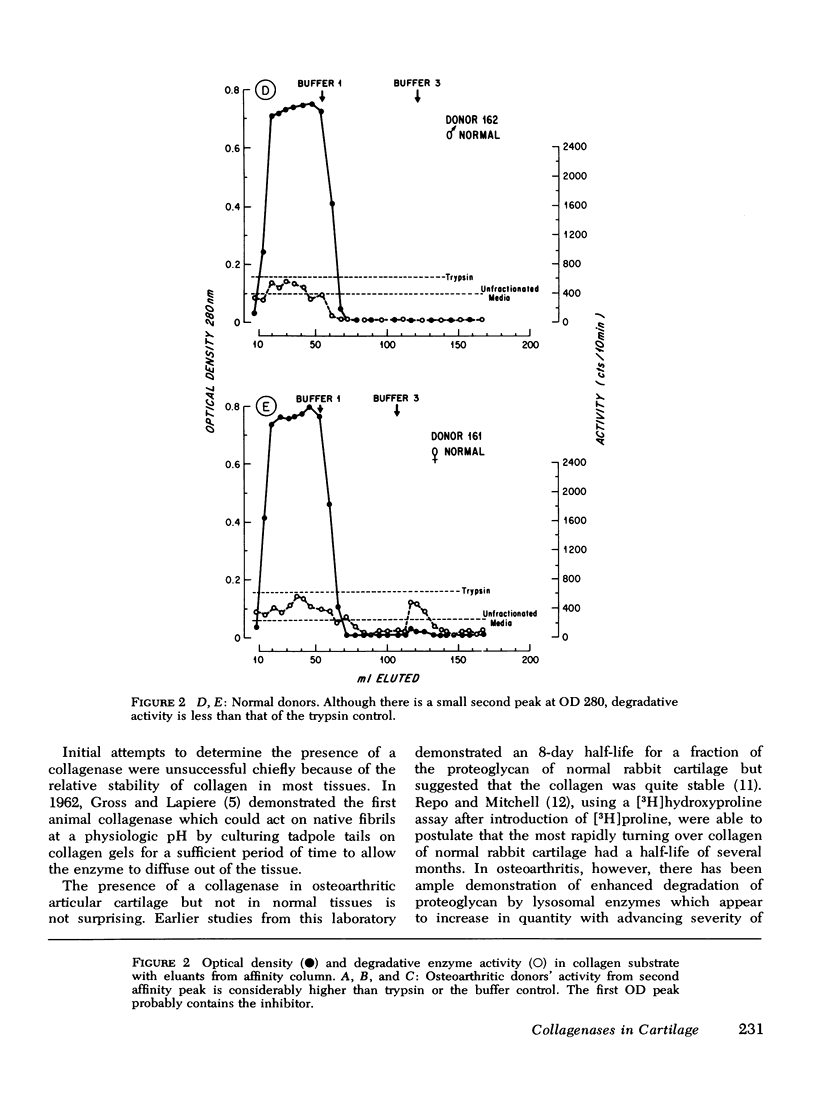
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ehrlich M. G., Mankin H. J., Treadwell B. V. Acid hydrolase activity in osteoarthritic and normal human cartilage. J Bone Joint Surg Am. 1973 Jul;55(5):1068–1076. [PubMed] [Google Scholar]
- Evanson J. M., Jeffrey J. J., Krane S. M. Human collagenase: identification and characterization of an enzyme from rheumatoid synovium in culture. Science. 1967 Oct 27;158(3800):499–502. doi: 10.1126/science.158.3800.499. [DOI] [PubMed] [Google Scholar]
- Evanson J. M., Jeffrey J. J., Krane S. M. Studies on collagenase from rheumatoid synovium in tissue culture. J Clin Invest. 1968 Dec;47(12):2639–2651. doi: 10.1172/JCI105947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullmer H. M., Gibson W. Collagenolytic activity in gingivae of man. Nature. 1966 Feb 12;209(5024):728–729. doi: 10.1038/209728a0. [DOI] [PubMed] [Google Scholar]
- GROSS J., LAPIERE C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang A. H., Nagai Y., Piez K. A., Gross J. Studies on the structure of collagen utilizing a collagenolytic enzyme from tadpole. Biochemistry. 1966 Feb;5(2):509–515. doi: 10.1021/bi00866a016. [DOI] [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Brown R. S., Bladen H. A., Fullmer H. M. Degradation of collagen by a human granulocyte collagenolytic system. J Clin Invest. 1968 Dec;47(12):2622–2629. doi: 10.1172/JCI105945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin H. J. The structure, chemistry and metabolism of articular cartilage. Bull Rheum Dis. 1967 Mar;17(7):447–452. [PubMed] [Google Scholar]
- Nagai Y. Vertebrate collagenase: further characterization and the significance of its latent form in vivo. Mol Cell Biochem. 1973 Jun 27;1(2):137–145. doi: 10.1007/BF01659325. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Repo R. U., Mitchell N. Collagen synthesis in mature articular cartilage of the rabbit. J Bone Joint Surg Br. 1971 Aug;53(3):541–548. [PubMed] [Google Scholar]
- Ryan J. N., Woessner J. F., Jr Mammalian collagenase: direct demonstration in homogenates of involuting rat uterus. Biochem Biophys Res Commun. 1971 Jul 2;44(1):144–149. doi: 10.1016/s0006-291x(71)80170-5. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Goldhaber P., Glimcher M. J. Further studies on the nature of the components in serum which inhibit mouse bone collagenase. Calcif Tissue Res. 1972;10(4):280–288. doi: 10.1007/BF02012559. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Sakamoto M., Goldhaber P., Glimcher M. J. Studies on the interaction between heparin and mouse bone collagenase. Biochim Biophys Acta. 1975 Mar 14;385(1):41–50. doi: 10.1016/0304-4165(75)90072-0. [DOI] [PubMed] [Google Scholar]
- Sapolsky A. I., Altman R. D., Woessner J. F., Howell D. S. The action of cathepsin D in human articular cartilage on proteoglycans. J Clin Invest. 1973 Mar;52(3):624–633. doi: 10.1172/JCI107224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J. F., BREWER T. H. FORMATION AND BREAKDOWN OF COLLAGEN AND ELASTIN IN THE HUMAN UTERUS DURING PREGNANCY AND POST-PARTUM INVOLUTION. Biochem J. 1963 Oct;89:75–82. doi: 10.1042/bj0890075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G., Lapiere C. M., Gross J. A collagenolytic factor in rat bone promoted by parathyroid extract. Biochem Biophys Res Commun. 1964 Apr 22;15(5):397–402. doi: 10.1016/0006-291x(64)90474-7. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr, Ryan J. N. Collagenase activity in homogenates of the involuting rat uterus. Biochim Biophys Acta. 1973 Jun 6;309(2):397–405. doi: 10.1016/0005-2744(73)90038-7. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Glanville R. W., Crossley M. J., Evanson J. M. Purification of rheumatoid synovial collagenase and its action on soluble and insoluble collagen. Eur J Biochem. 1975 Jun;54(2):611–622. doi: 10.1111/j.1432-1033.1975.tb04173.x. [DOI] [PubMed] [Google Scholar]





