Abstract
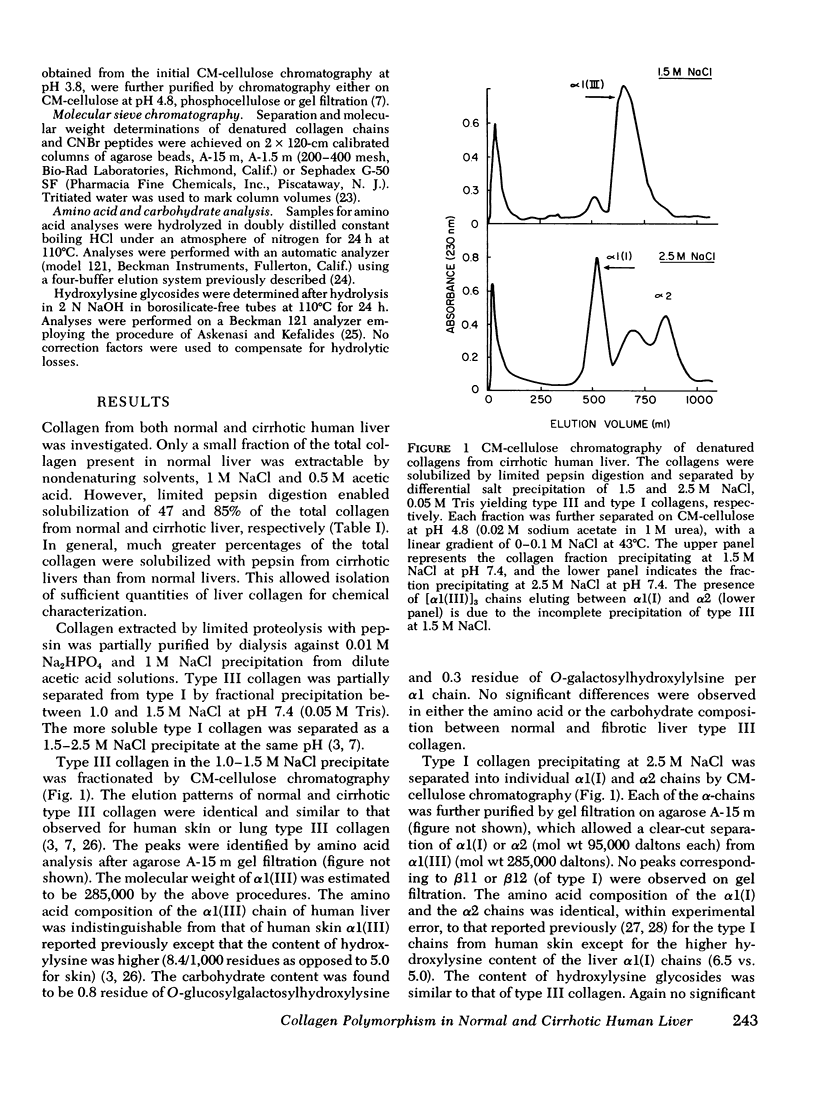
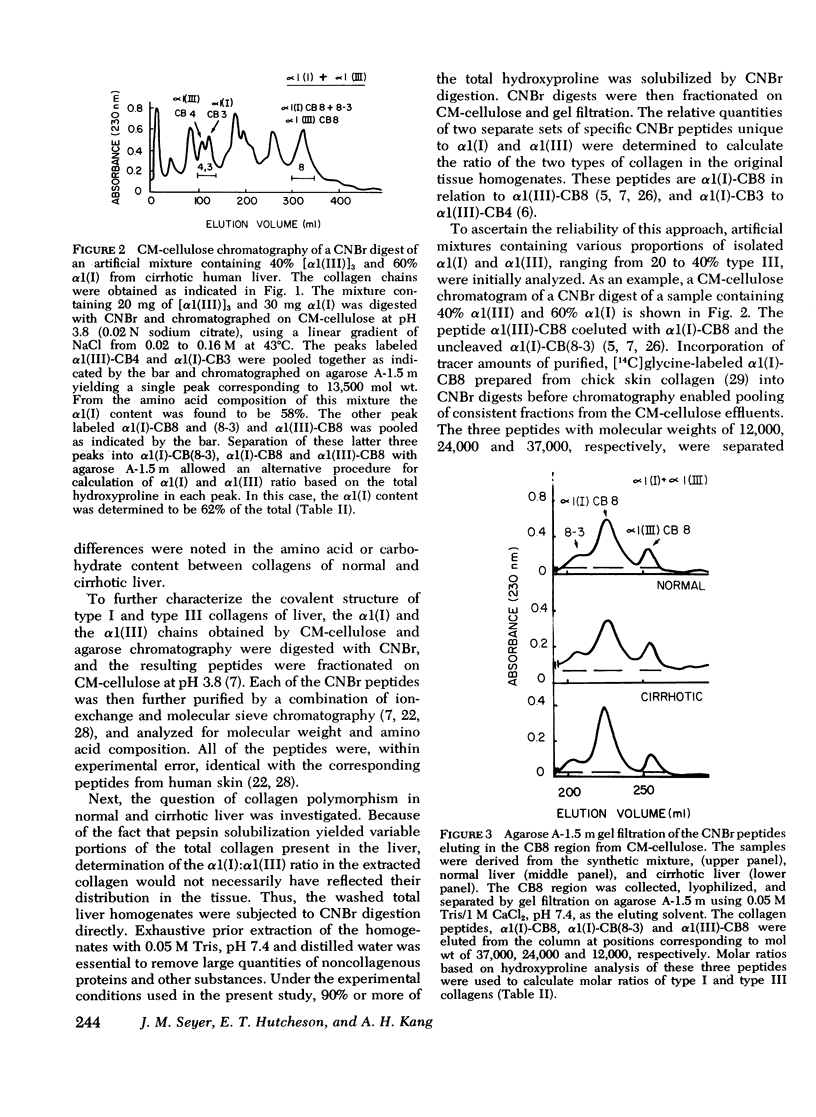
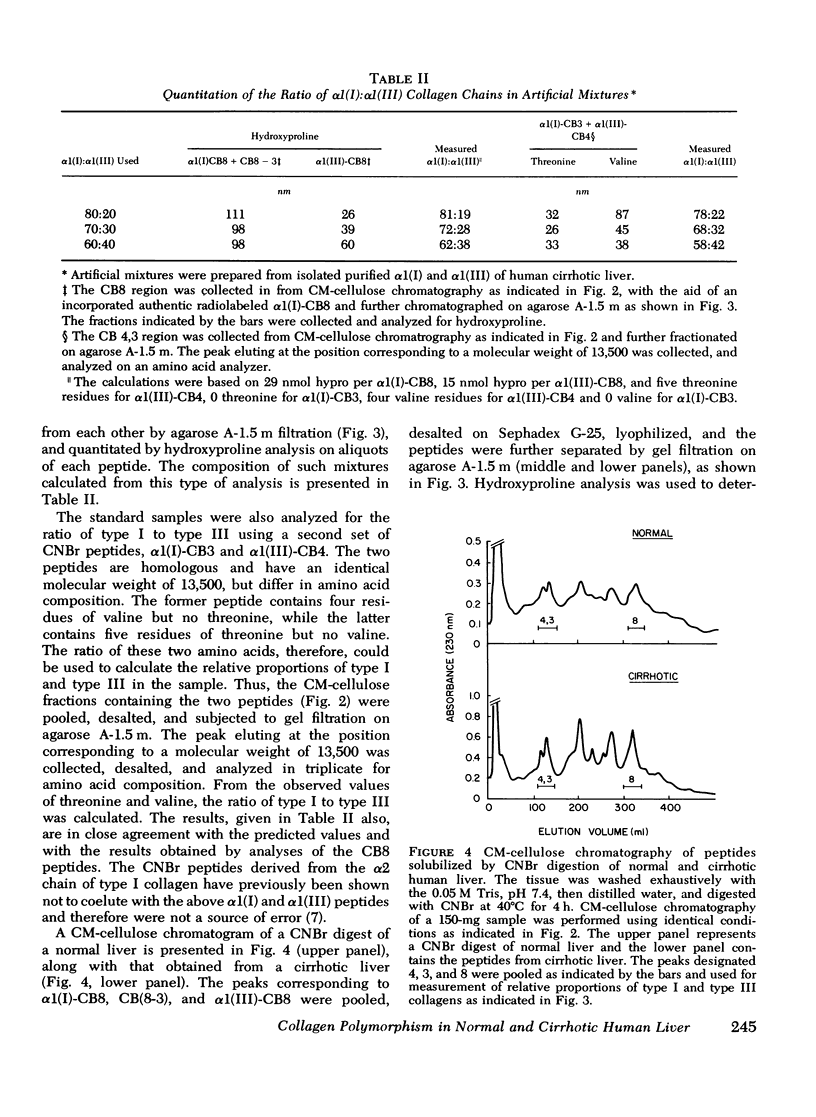
Collagens in normal human liver and in alcoholic cirrhotic liver were investigated. Collagens were solubilized by limited proteolysis with pepsin under nondenaturing conditions, and after purification, were fractionated into types I and III by selective precipitation with NaCl. After carboxymethyl cellulose and agarose chromatography, the resulting alpha-chains from each of the collagen types were analyzed with respect to their amino acid and carbohydrate compositions. A comparison of the results obtained from normal liver with those from the diseases organ revealed no significant differences. The isolated human liver alpha1(I) and alpha1(III) chains were digested with CNBr and the generated peptides were separated and purified by a combination of ion-exchange and molecular sieve chromatography. The molecular weight and the amino acid and the carbohydrate compositions of each of the peptides were identical to those of the corresponding human skin peptides except for the slightly higher content of hydroxylysine in some of the peptides. The relative content of type III in relation to type I collagen in both normal anc cirrhotic liver was determined by digesting washed liver homogenates directly with CNBr and quantitating the resultant alpha1(I) and alpha 1(III) peptides after chromatographic separation. The relative quantities of these peptides indicated that normal human liver contained an average of 47% type III, with the remainder being type I. Cirrhotic liver, on the other hand, contained a significantly smaller proportion of type III, ranging from 18 to 34% in different samples, with a corresponding increase in type I. These findings indicate that although the amino acid and carbohydrate compositions of collagens deposited in cirrhotic liver are normal, the fibrotic process of alcoholic liver disease in humans is accompanied by an alteration in tissue collagen polymorphism, and suggest that the observed alterations may have pathogenetic implications.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenasi R. S., Kefalides N. A. Simple chromatographic method for determination of 14 C-labeled lysine, hydroxylysine, and hydroxylysine glycosides. Anal Biochem. 1972 May;47(1):67–72. doi: 10.1016/0003-2697(72)90279-5. [DOI] [PubMed] [Google Scholar]
- BORNSTEIN P., PIEZ K. A. A BIOCHEMICAL STUDY OF HUMAN SKIN COLLAGEN AND THE RELATION BETWEEN INTRA- AND INTERMOLECULAR CROSS-LINKING. J Clin Invest. 1964 Sep;43:1813–1823. doi: 10.1172/JCI105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. T., Birkedal-Hansen H., Beegle W. F., Taylor R. E., Chung E. Proteins of the periodontium. Identification of collagens with the [alpha1(I)]2alpha2 and [alpha1(III)]3 structures in bovine periodontal ligament. J Biol Chem. 1975 Dec 10;250(23):8907–8912. [PubMed] [Google Scholar]
- Chen T. S., Leevy C. M. Collagen biosynthesis in liver disease of the alcoholic. J Lab Clin Med. 1975 Jan;85(1):103–112. [PubMed] [Google Scholar]
- Chung E., Keele E. M., Miller E. J. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(3) chain of human collagen. Biochemistry. 1974 Aug 13;13(17):3459–3464. doi: 10.1021/bi00714a006. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Epstein E. H., Jr, Munderloh N. H. Isolation and characterization of CNBr peptides of human (alpha 1 (III) )3 collagen and tissue distribution of (alpha 1 (I) )2 alpha 2 and (alpha 1 (III) )3 collagens. J Biol Chem. 1975 Dec 25;250(24):9304–9312. [PubMed] [Google Scholar]
- Epstein E. H., Jr, Scott R. D., Miller E. J., Piez K. A. Isolation and characterization of the peptides derived from soluble human and baboon skin collagen after cyanogen bromide cleavage. J Biol Chem. 1971 Mar 25;246(6):1718–1724. [PubMed] [Google Scholar]
- Feinman L., Lieber C. S. Hepatic collagen metabolism: effect of alcohol consumption in rats and baboons. Science. 1972 May 19;176(4036):795–795. doi: 10.1126/science.176.4036.795. [DOI] [PubMed] [Google Scholar]
- Gallop P. M., Blumenfeld O. O., Seifter S. Structure and metabolism of connective 801 tissue proteins. Annu Rev Biochem. 1972;41:617–672. doi: 10.1146/annurev.bi.41.070172.003153. [DOI] [PubMed] [Google Scholar]
- Gay S., Fietzek P. P., Remberger K., Eder M., Kühn K. Liver cirrhosis: immunofluorescence and biochemical studies demonstrate two types of collagen. Klin Wochenschr. 1975 Mar 1;53(5):205–208. doi: 10.1007/BF01468808. [DOI] [PubMed] [Google Scholar]
- HUTTERER F., RUBIN E., SINGER E. J., POPPER H. Alkalisoluble and insoluble collagen in infant, adult and cirrhotic livers. Proc Soc Exp Biol Med. 1959 Oct-Dec;102:534–536. doi: 10.3181/00379727-102-25309. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Piez K. A., Gross J. Characterization of the cyanogen bromide peptides from the alpha 1 chain of chick skin collagen. Biochemistry. 1969 Apr;8(4):1506–1514. doi: 10.1021/bi00832a029. [DOI] [PubMed] [Google Scholar]
- Kang A. H. Studies on the location of intermolecular cross-links in collagen. Isolation of a CNBr peptide containing -hydroxylysinonorleucine. Biochemistry. 1972 May 9;11(10):1828–1835. doi: 10.1021/bi00760a015. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation and characterization of cyanogen bromide peptides from basement membrane collagen. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1151–1158. doi: 10.1016/0006-291x(72)90955-2. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation of a collagen from basement membranes containing three identical - chains. Biochem Biophys Res Commun. 1971 Oct 1;45(1):226–234. doi: 10.1016/0006-291x(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of the cyanogen bromide peptides from the l(II) chain of chick cartilage collagen. Biochemistry. 1971 Aug 3;10(16):3030–3035. doi: 10.1021/bi00792a007. [DOI] [PubMed] [Google Scholar]
- POPPER H., PARONETTO F., SCHAFFNER F., PEREZ V. Studies on hepatic fibrosis. Lab Invest. 1961 Mar-Apr;10:265–290. [PubMed] [Google Scholar]
- Piez K. A. Molecular weight determination of random coil polypeptides from collagen by molecular sieve chromatography. Anal Biochem. 1968 Nov;26(2):305–312. doi: 10.1016/0003-2697(68)90342-4. [DOI] [PubMed] [Google Scholar]
- Popper H., Uenfriend S. Hepatic fibrosis. Correlation of biochemical and morphologic investigations. Am J Med. 1970 Nov;49:707–721. doi: 10.1016/s0002-9343(70)80135-8. [DOI] [PubMed] [Google Scholar]
- Remberger K., Gay S., Fietzek P. P. Immunohistochemische Untersuchungen zur Kollagencharakterisierung in Lebercirrhosen. Virchows Arch A Pathol Anat Histol. 1975 Aug 12;367(3):231–240. doi: 10.1007/BF00430710. [DOI] [PubMed] [Google Scholar]
- Risteli J., Kivirikko K. I. Activities of prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase and collagen glucosyltransferase in the liver of rats with hepatic injury. Biochem J. 1974 Oct;144(1):115–122. doi: 10.1042/bj1440115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojkind M., Martinez-Palomo A. Increase in type I and type III collagens in human alcoholic liver cirrhosis. Proc Natl Acad Sci U S A. 1976 Feb;73(2):539–543. doi: 10.1073/pnas.73.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyer J. M., Hutcheson E. T., Kang A. H. Collagen polymorphism in idiopathic chronic pulmonary fibrosis. J Clin Invest. 1976 Jun;57(6):1498–1507. doi: 10.1172/JCI108420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth C. A., Forrest L. Changes in guinea-pig dermal collagen during development. Eur J Biochem. 1975 Jul 1;55(2):391–395. doi: 10.1111/j.1432-1033.1975.tb02174.x. [DOI] [PubMed] [Google Scholar]
- Shuttleworth C. A., Forrest L. Pepsin-solubilized collagens of guinea-pig dermis and dermal scar. Biochim Biophys Acta. 1974 Oct 9;365(2):454–457. doi: 10.1016/0005-2795(74)90023-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Prockop D. J. Protocollagen proline hydroxylase in normal liver and in hepatic fibrosis. Gastroenterology. 1969 Apr;56(4):744–750. [PubMed] [Google Scholar]
- Trelstad R. L. Human aorta collagens: evidence for three distinct species. Biochem Biophys Res Commun. 1974 Apr 8;57(3):717–725. doi: 10.1016/0006-291x(74)90605-6. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Toole B. P., Gross J. Collagen heterogeneity. High resolution separation of native ( 1(I) 2 2 and ( 1(II) 3 and their component chains. J Biol Chem. 1972 Oct 25;247(20):6469–6473. [PubMed] [Google Scholar]
- Weiss J. B., Shuttleworth C. A., Brown R., Sedowfia K., Baildam A., Hunter J. A. Occurrence of type III collagen in inflamed synovial membranes: a comparison between non rheumatoid, rheumatoid, and normal synovial collagens. Biochem Biophys Res Commun. 1975 Aug 4;65(3):907–912. doi: 10.1016/s0006-291x(75)80471-2. [DOI] [PubMed] [Google Scholar]



