Abstract
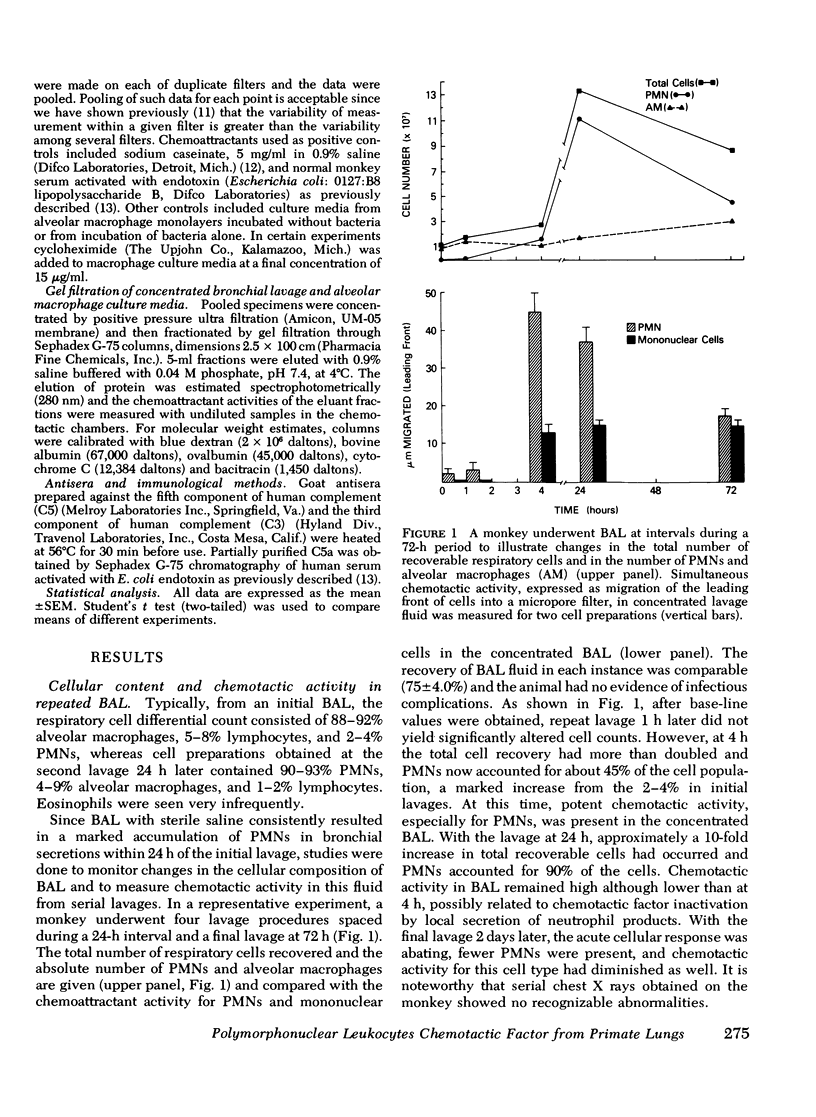
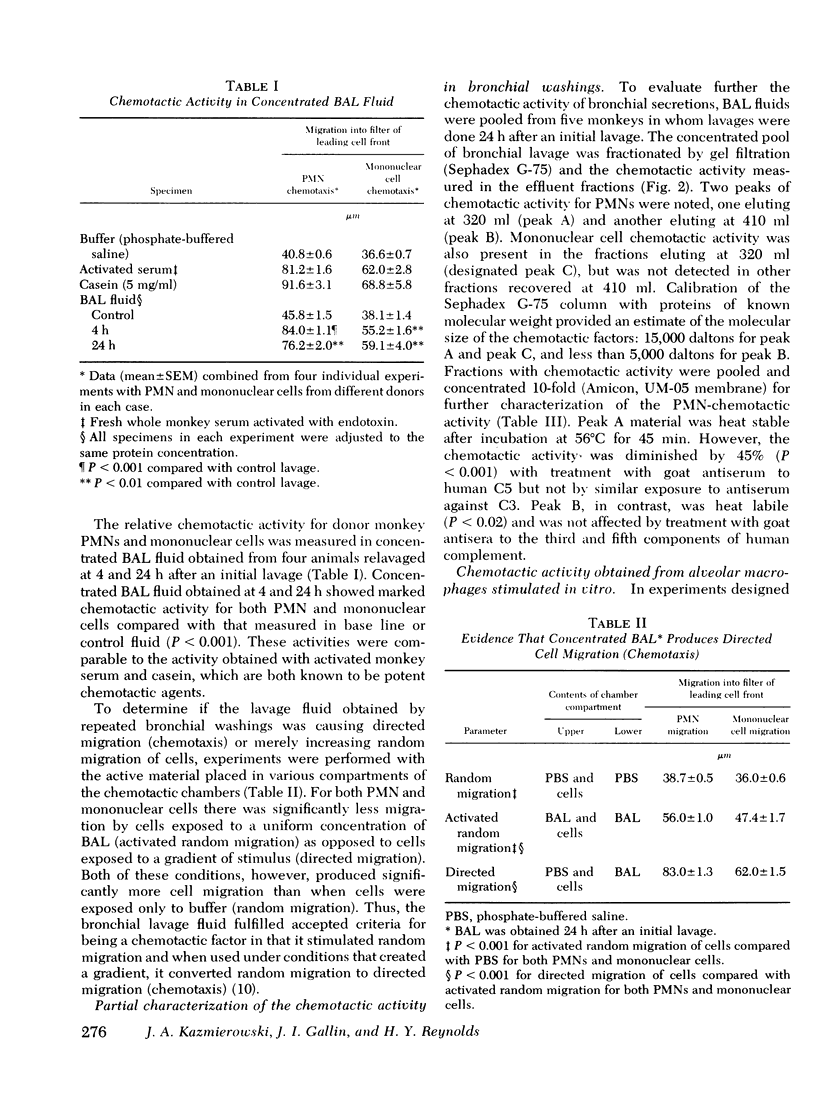
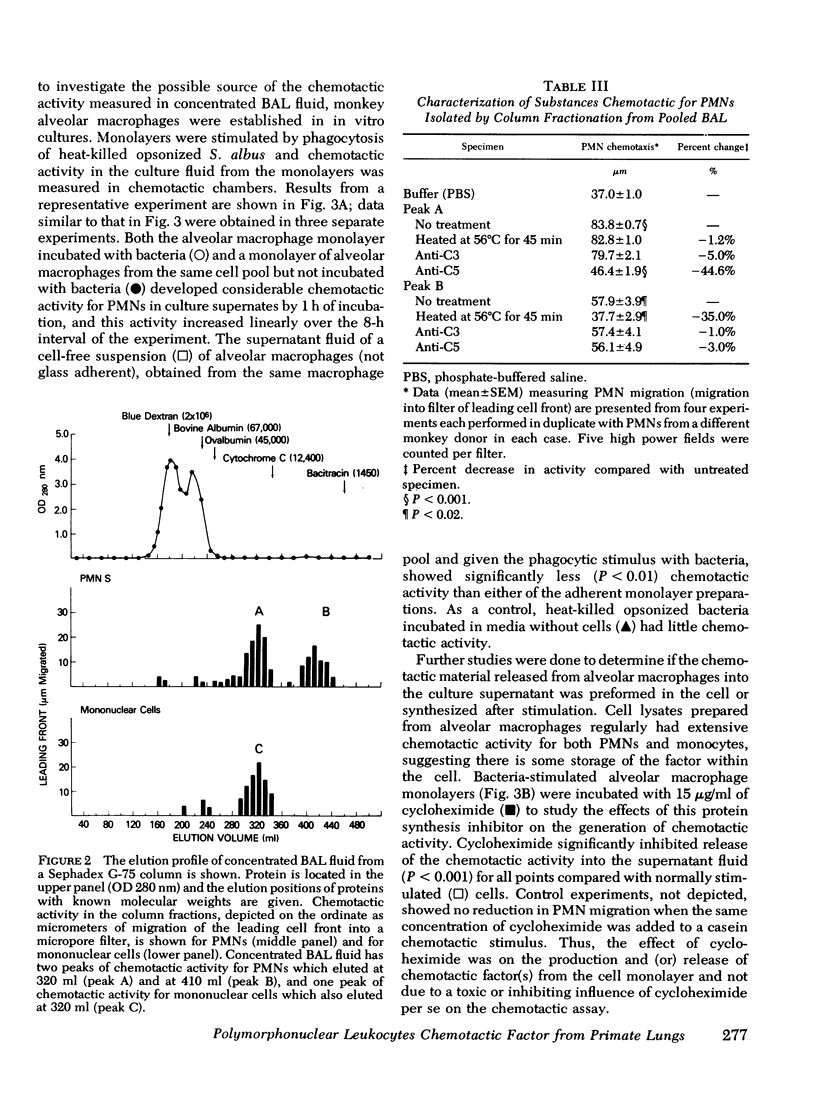
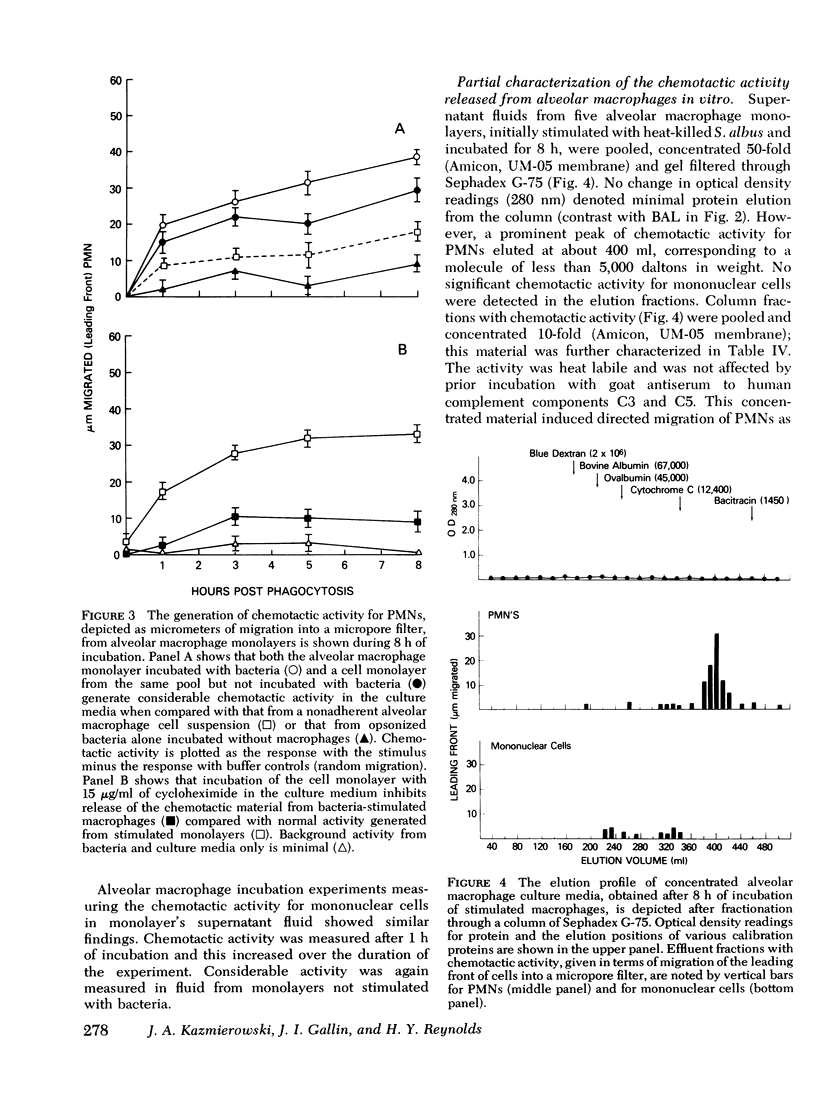
Approximately 4 h after an initial bronchoalveolar lavage (BAL) of a primate's lung, an appreciable number of polymorphonuclear leukocytes (PMNs) were noted to accumulate in respiratory fluids when lavage was repeated. Whereas, alveolar macrophages (90%) and lymphocytes (7%) were the principal respiratory cells recovered initially from lavage fluid, later samples contained 45-90% PMNs To explain the observed ingress of PMNs into lung fluids, concentrated BAL fluid was tested for chemoattractant activity. Such fluid obtained 4 and 24 h after an initial lavage contained material that produced directed migration (chemotaxis) for PMNs and mononuclear cells isolated from peripheral blood of normal donors. Gel filtration chromatography of BAL disclosed two peaks of chemotactic activity in the effluent fractions. Material from the column with an estimated molecular weight of 15,000 daltons was chemotactic for both PMNs and mononuclear cells. Because it was susceptible to inactivation with antiserum against the fifth component of complement, resistant to heating, and unaffected by antiserum against C3, this factor was considered analogous to the cleavage product of the fifth component of complement. C5a. In addition chemotactic activity for PMNs only was contained in an effluent peak having a molecular weight of about 5,000 daltons. This material was heat labile but unaffected by antisera to complement components. To locate the possible source of these factors in respiratory fluid, in vitro cultures of alveolar macrophages were established. These cells, whether stimulated by phagocytosis of opsonized bacteria or merely by attachment to a glass surface, produced chemotactic material which had physical characteristics similar to the small molecular weight material in BAL. Moreover, it induced preferential chemotaxis for PMNs. Thus, in primate lungs, at least two chemotactic substances may generate an inflammatory response; one which is a fragment of the complement component C5 and another small molecular weight factor which is released from alveolar macrophages.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodel P. Studies on the mechanism of endogenous pyrogen production. III. Human blood monocytes. J Exp Med. 1974 Oct 1;140(4):954–965. doi: 10.1084/jem.140.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Keller H. U., Sorkin E. Studies on chemotaxis. XI. Effect on neutrophils of lysosomal and other subcellular fractions from leukocytes. Int Arch Allergy Appl Immunol. 1969;35(2):194–205. [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Gallin J. I., Kaplan A. P. The selective eosinophil chemotactic activity of histamine. J Exp Med. 1975 Dec 1;142(6):1462–1476. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Kimball H. R. Defective granulocyte chemotaxis in the Chediak-Higashi syndrome. J Clin Invest. 1971 Dec;50(12):2645–2652. doi: 10.1172/JCI106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicetti L., Colombo B., Baglioni C. Inhibition of protein synthesis in reticulocytes by antibiotics. II. The site of action of cycloheximide, streptovitacin A and pactamycin. Biochim Biophys Acta. 1966 Apr 18;119(1):120–129. [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Frank M. M. Kinetic analysis of chemotactic factor generation in human serum via activation of the classical and alternate complement pathways. Clin Immunol Immunopathol. 1975 Jan;3(3):334–346. doi: 10.1016/0090-1229(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Kirkpatrick C. H. Chemotactic activity in dialyzable transfer factor. Proc Natl Acad Sci U S A. 1974 Feb;71(2):498–502. doi: 10.1073/pnas.71.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. Purification and synthesis of eosinophilotactic tetrapeptides of human lung tissue: identification as eosinophil chemotactic factor of anaphylaxis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4123–4127. doi: 10.1073/pnas.72.10.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayzel A. I., Fuhr J. E., London I. M. Effects of inhibitors of protein synthesis on the synthesis of heme in rabbit reticulocytes. Biochem Biophys Res Commun. 1967 Sep 7;28(5):705–710. doi: 10.1016/0006-291x(67)90373-7. [DOI] [PubMed] [Google Scholar]
- Green G. M. The J. Burns Amberson Lecture--in defense of the lung. Am Rev Respir Dis. 1970 Nov;102(5):691–703. doi: 10.1164/arrd.1970.102.5.691. [DOI] [PubMed] [Google Scholar]
- HANKS J. H., WALLACE J. H. Determination of cell viability. Proc Soc Exp Biol Med. 1958 May;98(1):188–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- Kazmierowski J. A., Fauci A. S., Reynolds H. Y. Characterization of lymphocytes in bronchial lavage fluid from monkeys. J Immunol. 1976 Mar;116(3):615–618. [PubMed] [Google Scholar]
- Keller H. U., Sorkin E. Chemotaxis of leucocytes. Experientia. 1968 Jul 15;24(7):641–652. doi: 10.1007/BF02138287. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. I. Analysis of protein and lipids in bronchial secretions and antibody responses after vaccination with pseudomonas aeruginosa. J Immunol. 1973 Aug;111(2):358–368. [PubMed] [Google Scholar]
- Schorlemmer H. U., Davies P., Allison A. C. Ability of activated complement components to induce lysosomal enzyme release from macrophages. Nature. 1976 May 6;261(5555):48–49. doi: 10.1038/261048a0. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Snyderman R., Phillips J., Mergenhagen S. E. Polymorphonuclear leukocyte chemotactic activity in rabbit serum and Guinea pig serum treated with immune complexes: evidence for c5a as the major chemotactic factor. Infect Immun. 1970 Jun;1(6):521–525. doi: 10.1128/iai.1.6.521-525.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher V. J., Morse J. H., Thorbecke G. J. Sites of production of primate serum proteins associated with complement system. Proc Soc Exp Biol Med. 1967 Feb;124(2):433–438. doi: 10.3181/00379727-124-31758. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD W. B., Jr Studies on the cellular immunology of acute bacterial infections. Harvey Lect. 1951;Series 47:72–98. [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by endotoxin-activated macrophages. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3598–3601. doi: 10.1073/pnas.71.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970 Mar;104(3):535–543. [PubMed] [Google Scholar]
- Ward P. A. Leukotaxis and leukotactic disorders. A review. Am J Pathol. 1974 Dec;77(3):520–538. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Quantitative phagocytosis by neutrophils. II. Release of the C5-cleaving enzyme and inhibition of phagocytosis by rheumatoid factor. J Immunol. 1973 Dec;111(6):1777–1782. [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



