Abstract
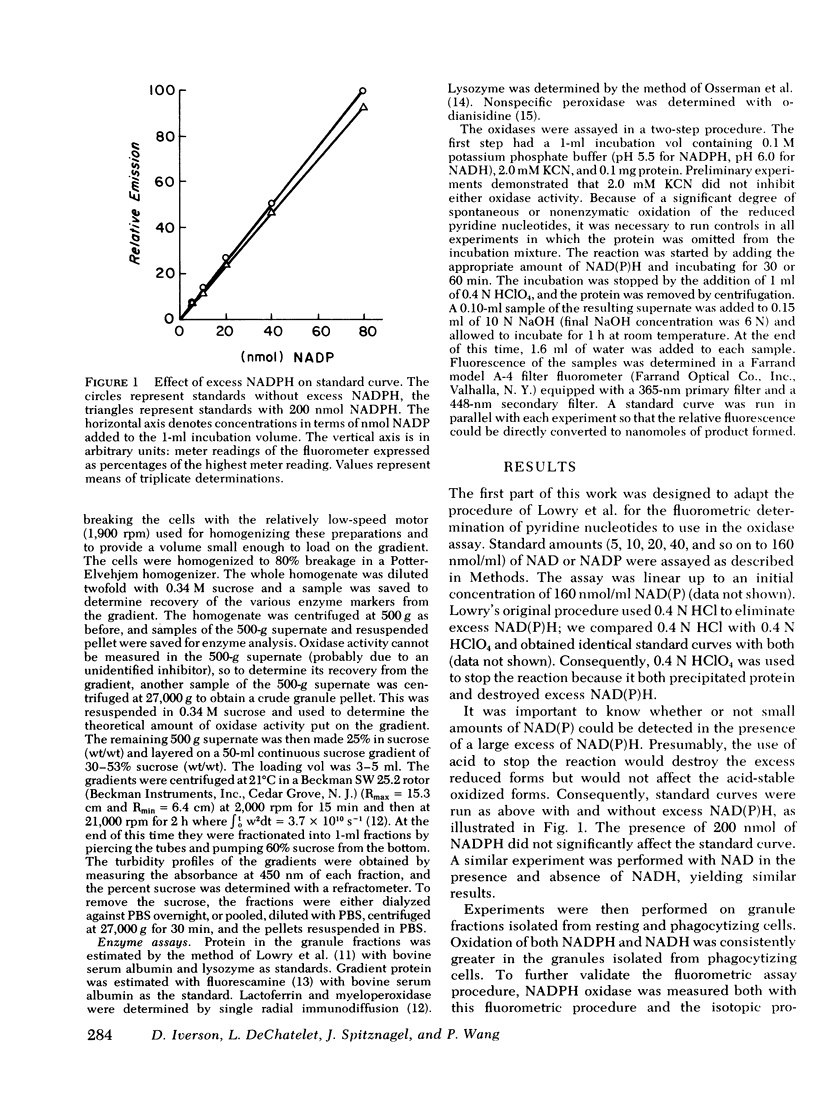
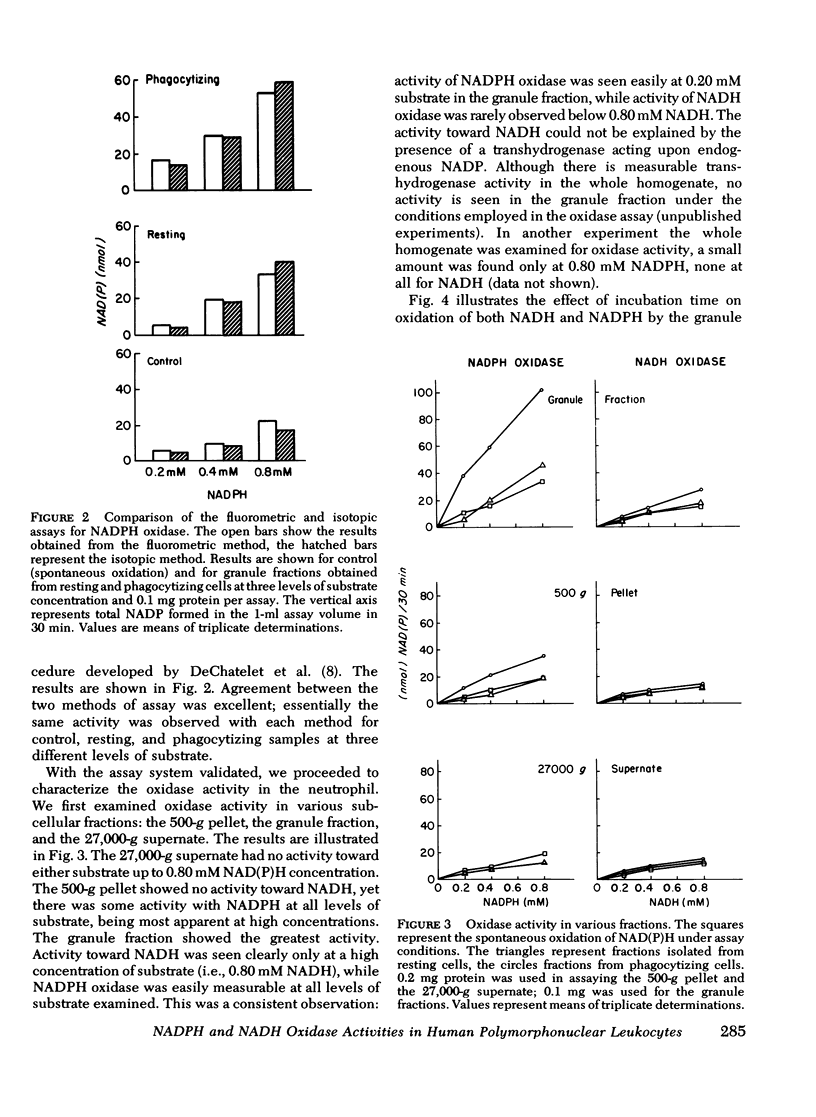
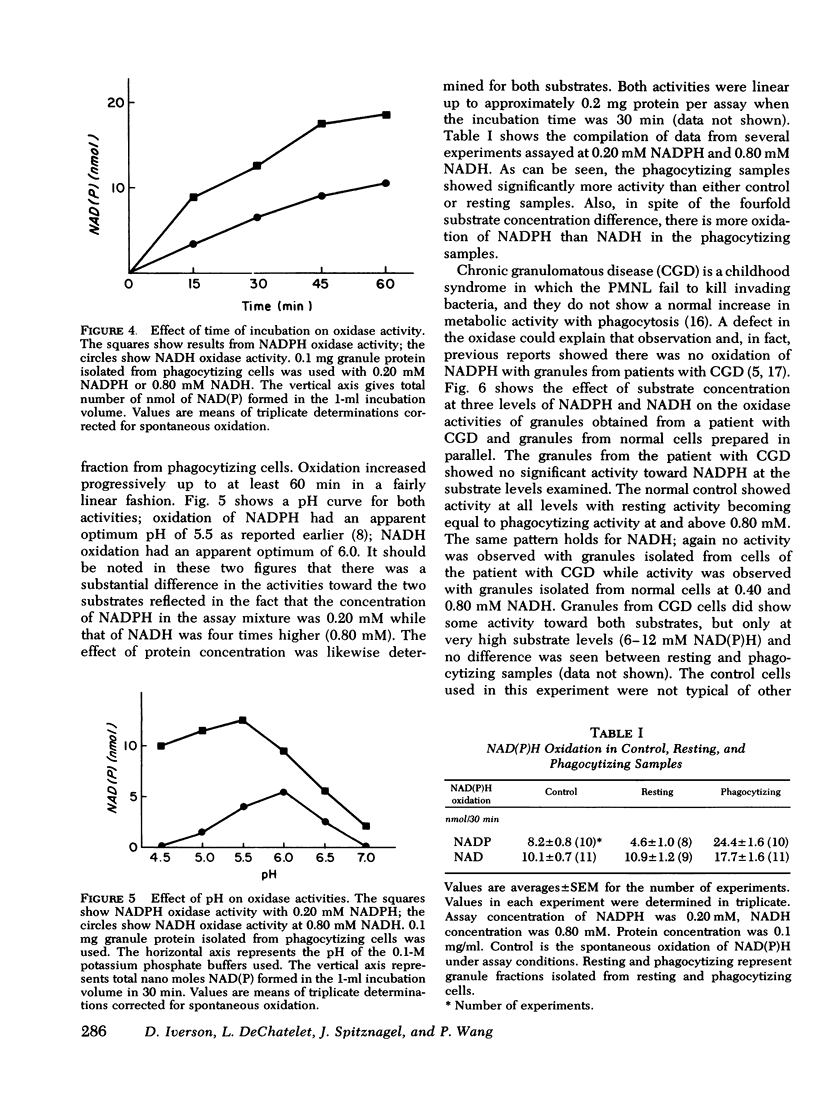
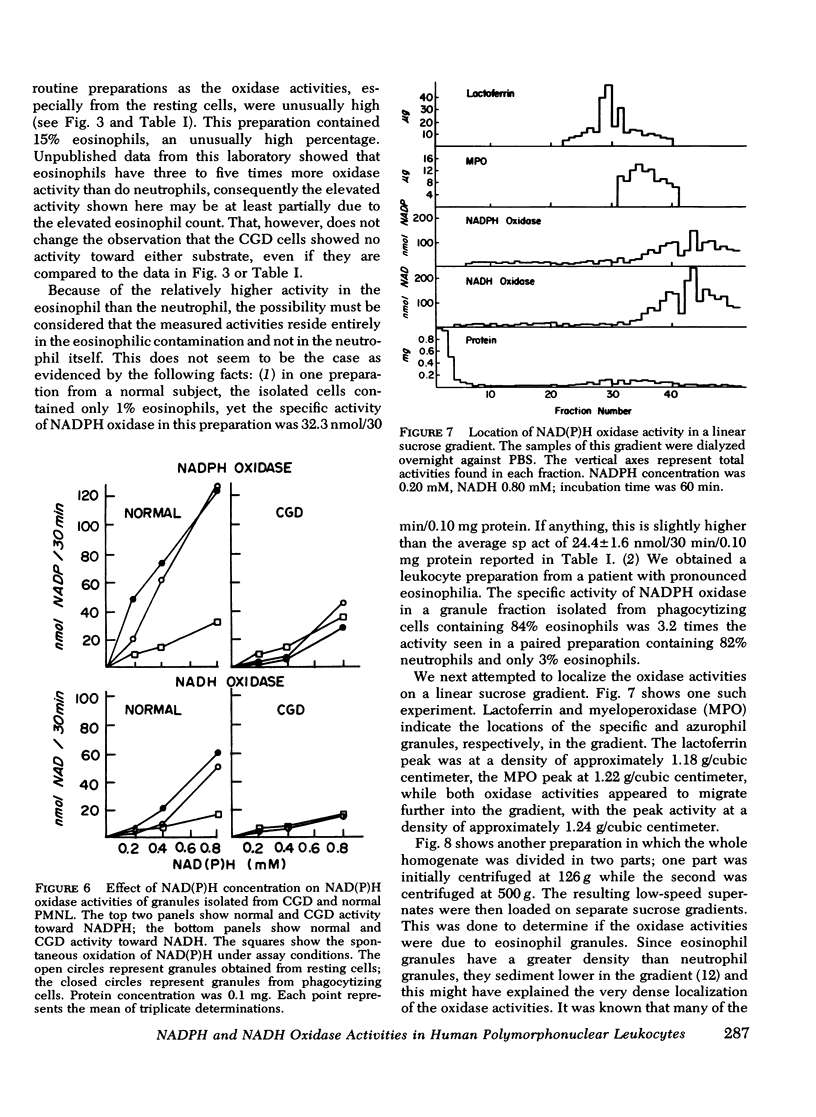
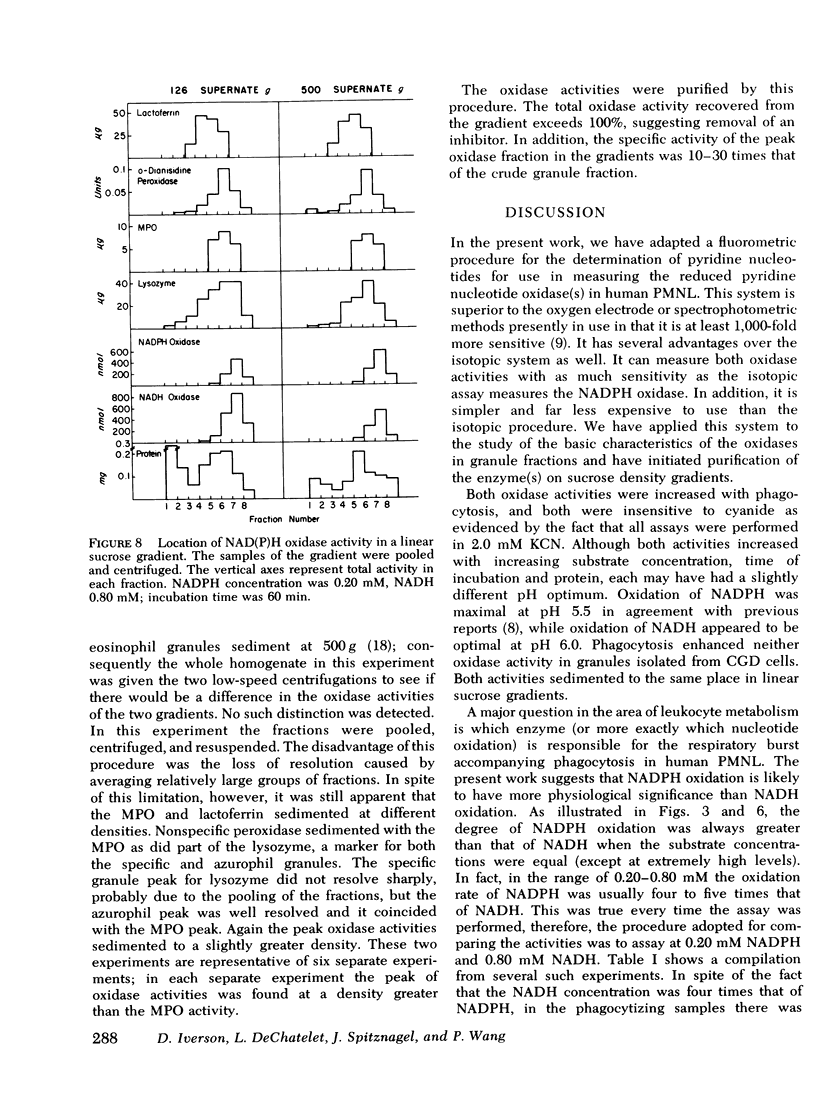
A fluormetric method for the determination of pyridine nucleotides has been adapted for use in studying the reduced pyridine nucleotide oxidases in human polymorphonuclear leukocytes. In the presence of strong base the oxidized forms of the pyridine nucleotides form a highly fluorescent product. The small amounts of NAD(P) formed by the oxidase reactions can be determined with great sensitivity. This method has been compared to the radioisotopic assay for NADPH oxidation. Both methods gave essentially the same results in terms of nanomoles NADP produced by control, resting, and phagocytizing samples. Both NADPH and NADH oxidase activities were insensitive to cyanide. NADPH oxidation had a pH optimum of 5.5, while that for NADH appeared to be 6.0. Granules isolated from phagocytizing cells routinely showed more activity toward both substrates (two to threefold) than granules from resting cells. Both activities were located primarily in a granule fraction prepared by differential centrifugation. Oxidation of NADPH was routinely four to five times that of NADH at all except very high substrate levels. Measurable NADH oxidation was rarely seen below 0.80 mM NADH, while NADPH oxidation was easily measurable at 0.20 mM. One patient with chronic granulomatous disease was studied. At low substrate levels, there was no activity toward either substrate in granules isolated from either resting or phagocytizing cells of this patient, while granules isolated from normal control cells showed substantial activity at these substrate levels. Purification of the activities had been initiated with linear sucrose gradients. Both activities co-sediment to a very dense region of the gradient, a region different from that in which membrane or azurophil granules usually equilibrate. The peak gradient fractions show a 10-30-fold increase in specific activity over comparable granule fractions. These data suggest that the oxidase activities are associated with one enzyme that has different affinities for the two substrates ans support the contention that the oxidation of NADPH is responsible for the metabolic burst accompanying phagocytosis in human PMNL.
Full text
PDF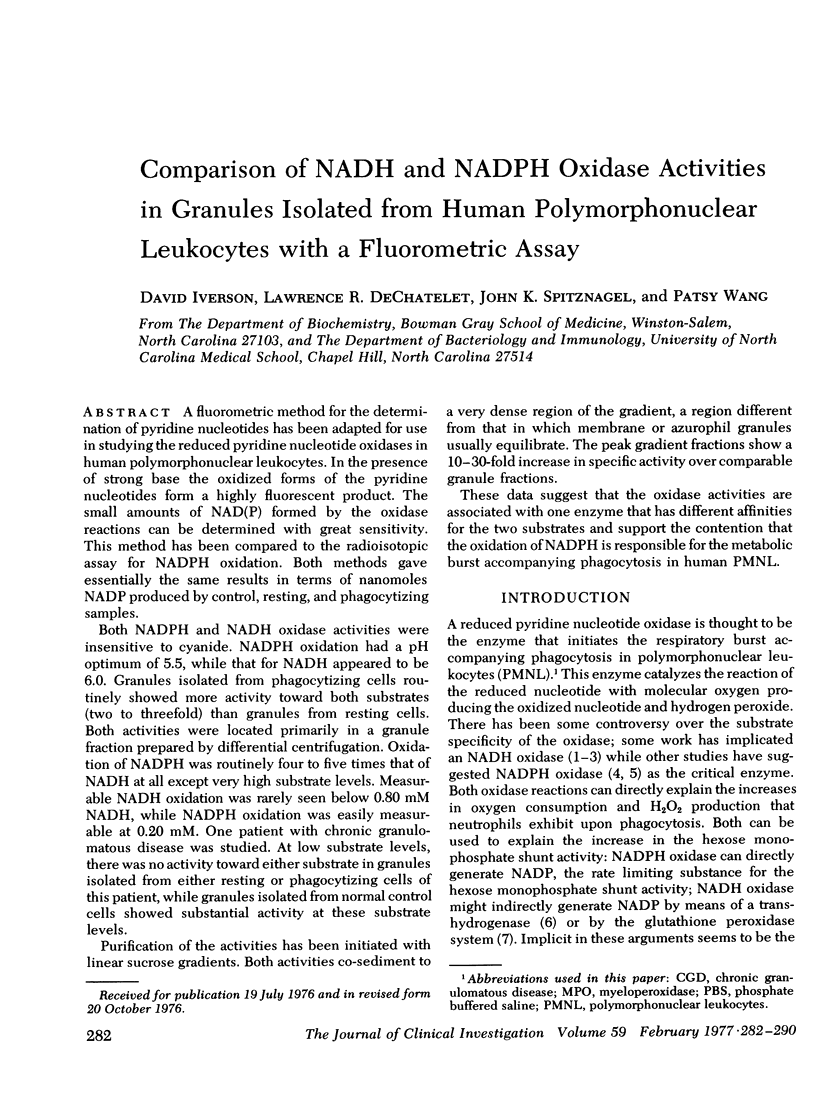



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKAZAWA T., CONN E. E. The oxidation of reduced pyridine nucleotides by peroxidase. J Biol Chem. 1958 May;232(1):403–415. [PubMed] [Google Scholar]
- ARCHER G. T., HIRSCH J. G. ISOLATION OF GRANULES FROM EOSINOPHIL LEUCOCYTES AND STUDY OF THEIR ENZYME CONTENT. J Exp Med. 1963 Aug 1;118:277–286. doi: 10.1084/jem.118.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Karnovsky M. L. Deficiency of reduced nicotinamide-adenine dinucleotide oxidase in chronic granulomatous disease. Science. 1968 Dec 13;162(3859):1277–1279. doi: 10.1126/science.162.3859.1277. [DOI] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- CAGAN R. H., KARNOVSKY M. L. ENZYMATIC BASIS OF THE RESPIRATORY STIMULATION DURING PHAGOCYTOSIS. Nature. 1964 Oct 17;204:255–257. doi: 10.1038/204255a0. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., McPhail L. C., Mullikin D., McCall C. E. An isotopic assay for NADPH oxidase activity and some characteristics of the enzyme from human polymorphonuclear leukocytes. J Clin Invest. 1975 Apr;55(4):714–721. doi: 10.1172/JCI107981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., McPhail L. C., Mullikin D., McCall C. E. Reduced nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide phosphate diaphorase activity in human polymorphonuclear leukocytes. Infect Immun. 1974 Sep;10(3):528–534. doi: 10.1128/iai.10.3.528-534.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. E., Kaplan N. O. Pyridine nucleotide transhydrogenase in normal human and leukemic leukocytes. J Clin Invest. 1966 Aug;45(8):1268–1272. doi: 10.1172/JCI105433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn D. C., Lehrer R. I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975 Apr;55(4):707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN N. O., COLOWICK S. P., BARNES C. C. Effect of alkali on diphosphopyridine nucleotide. J Biol Chem. 1951 Aug;191(2):461–472. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., KAPPHAHN J. I. The fluorometric measurement of pyridine nucleotides. J Biol Chem. 1957 Feb;224(2):1047–1064. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Dri P., Fant L., Basford R. E., Rossi F. NADPH oxidizing activity in rabbit polymorphonuclear leukocytes: localization in azurophilic granules. Biochem Biophys Res Commun. 1973 Aug 6;53(3):830–837. doi: 10.1016/0006-291x(73)90168-x. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- ROBERTS J., QUASTEL J. H. OXIDATION OF REDUCED TRIPHOSPHOPYRIDINE NUCLEOTIDE BY GUINEA PIG POLYMORPHONUCLEAR LEUCOCYTES. Nature. 1964 Apr 4;202:85–86. doi: 10.1038/202085a0. [DOI] [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- Segal A. W., Peters T. J. Characterisation of the enzyme defect in chronic granulomatous disease. Lancet. 1976 Jun 26;1(7974):1363–1365. doi: 10.1016/s0140-6736(76)93021-x. [DOI] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Wilkinson R. W., Powars D. R., Hochstein P. New evidence for the role of NADH oxidase in phagocytosis by human granulocytes. Biochem Med. 1975 May;13(1):83–88. doi: 10.1016/0006-2944(75)90142-8. [DOI] [PubMed] [Google Scholar]



