Abstract
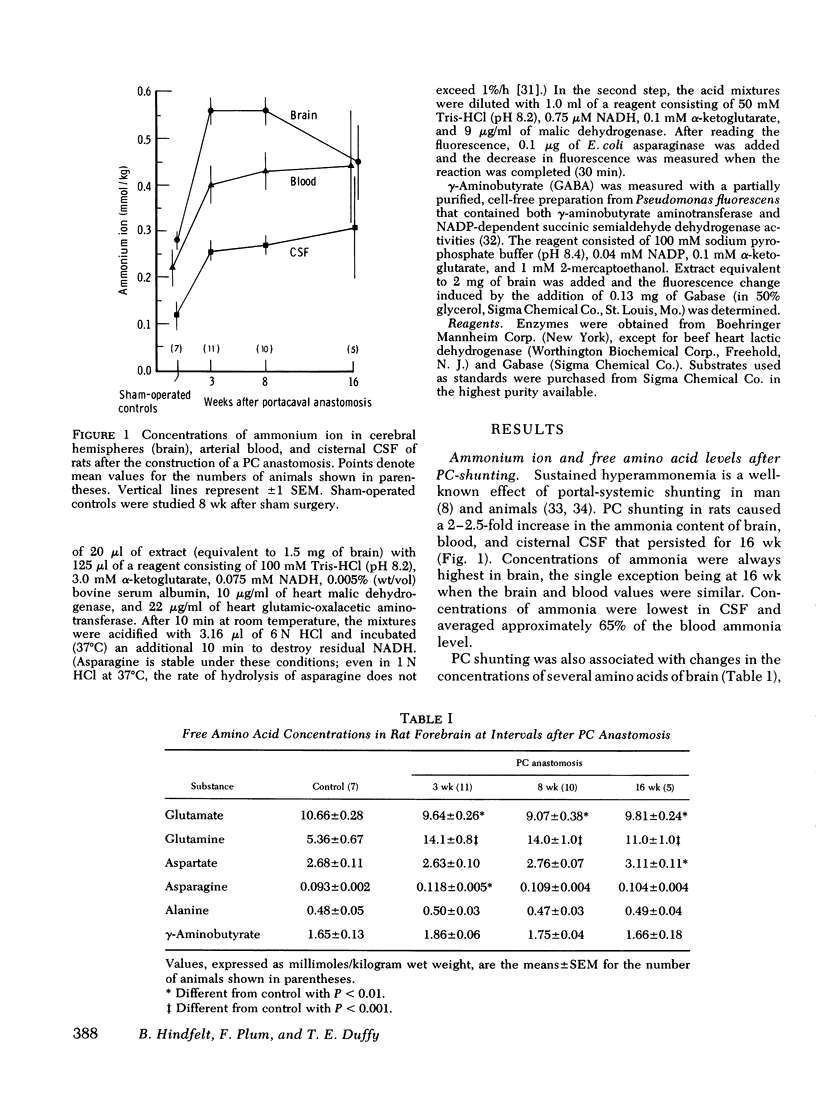
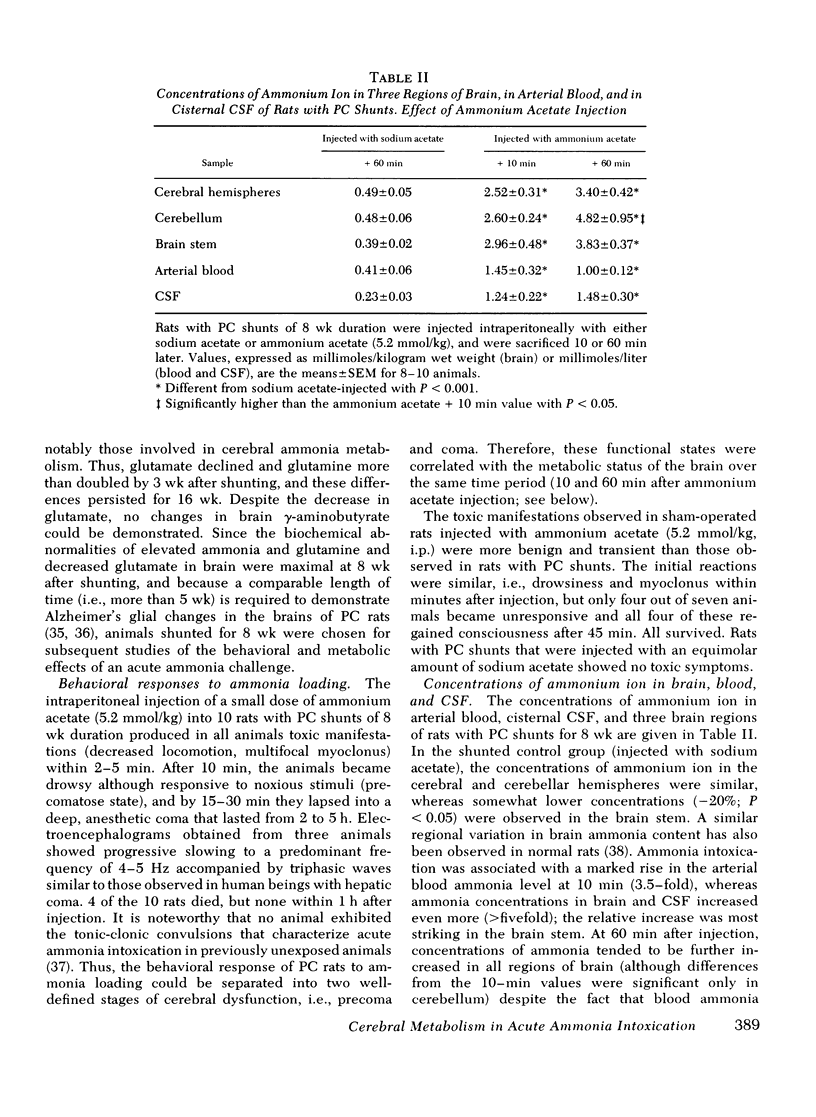
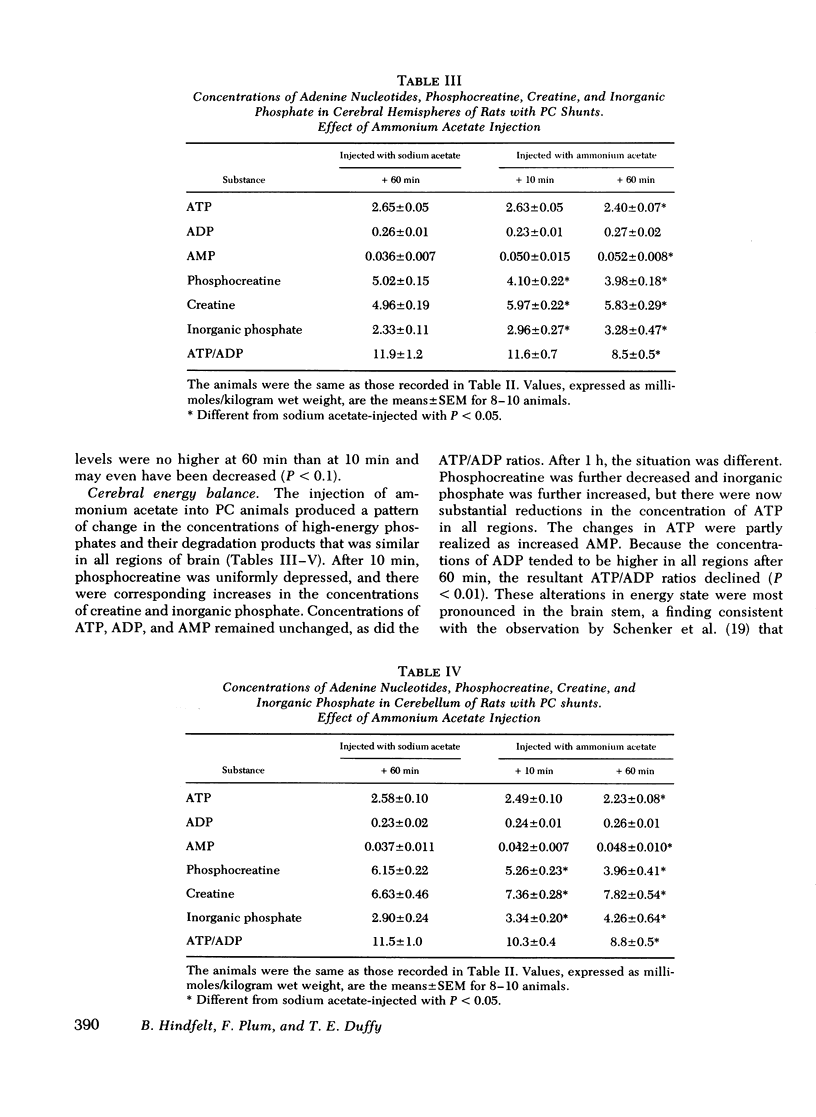
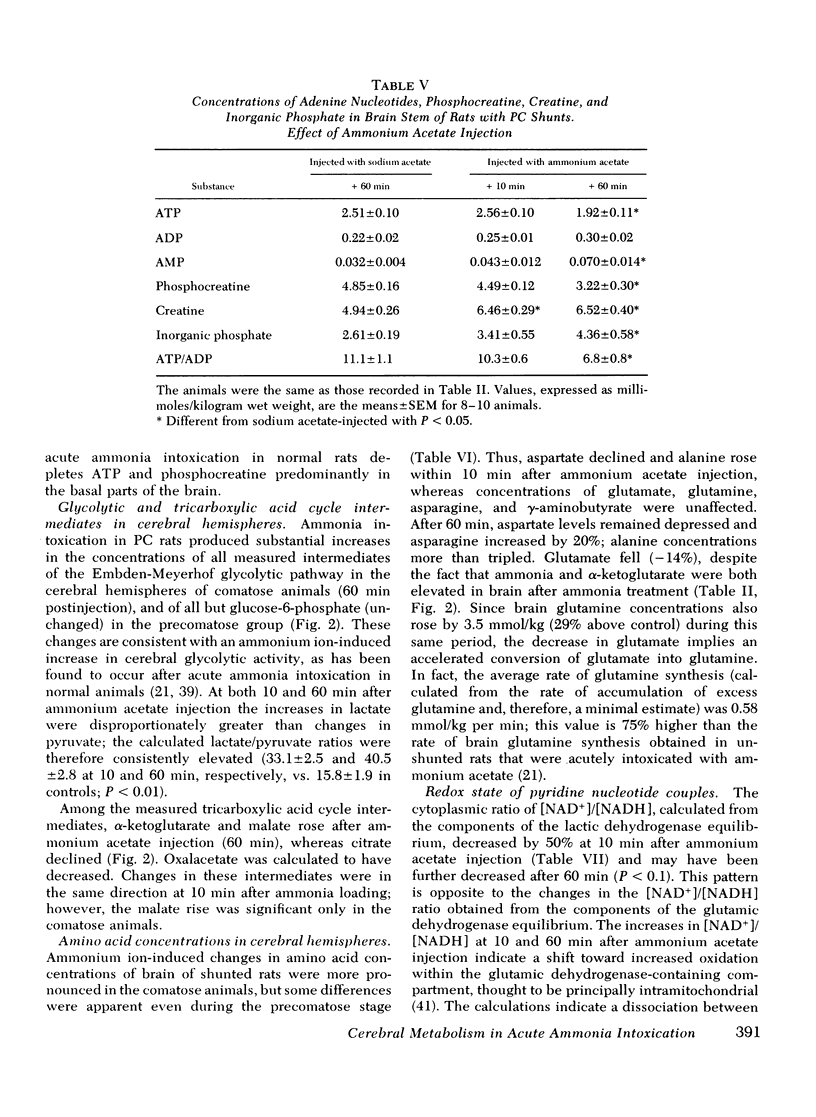
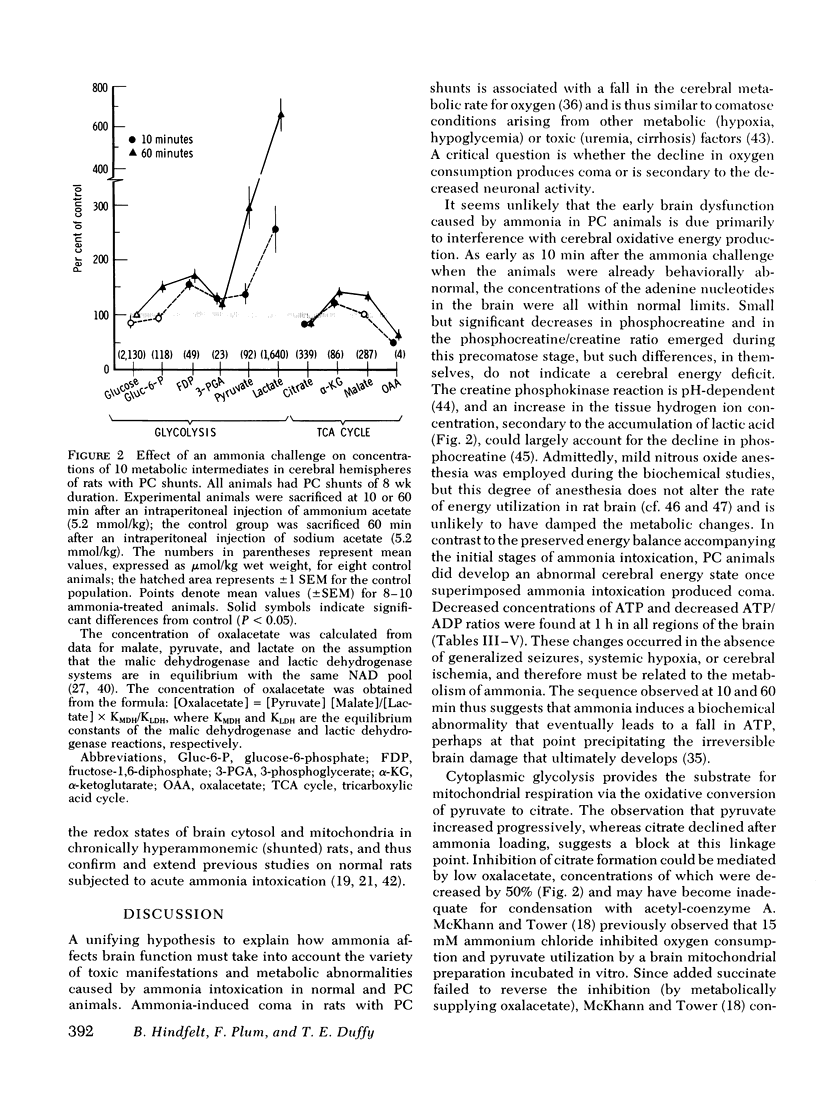
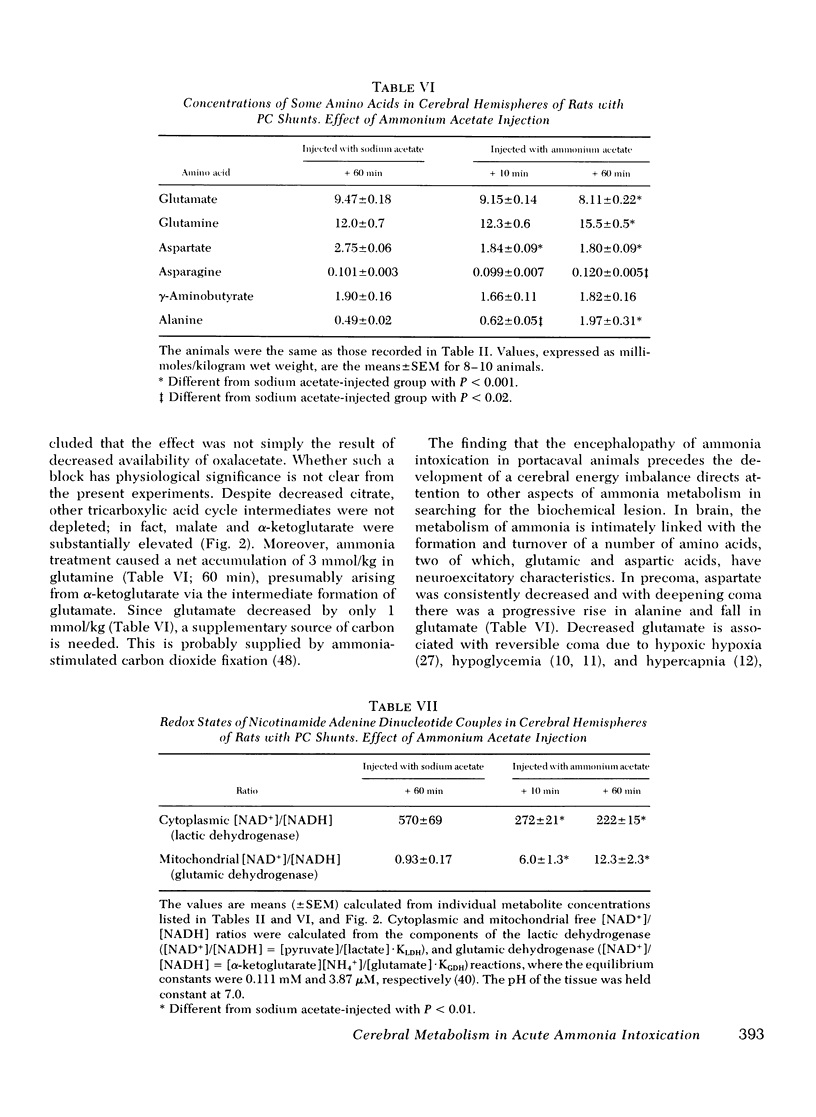
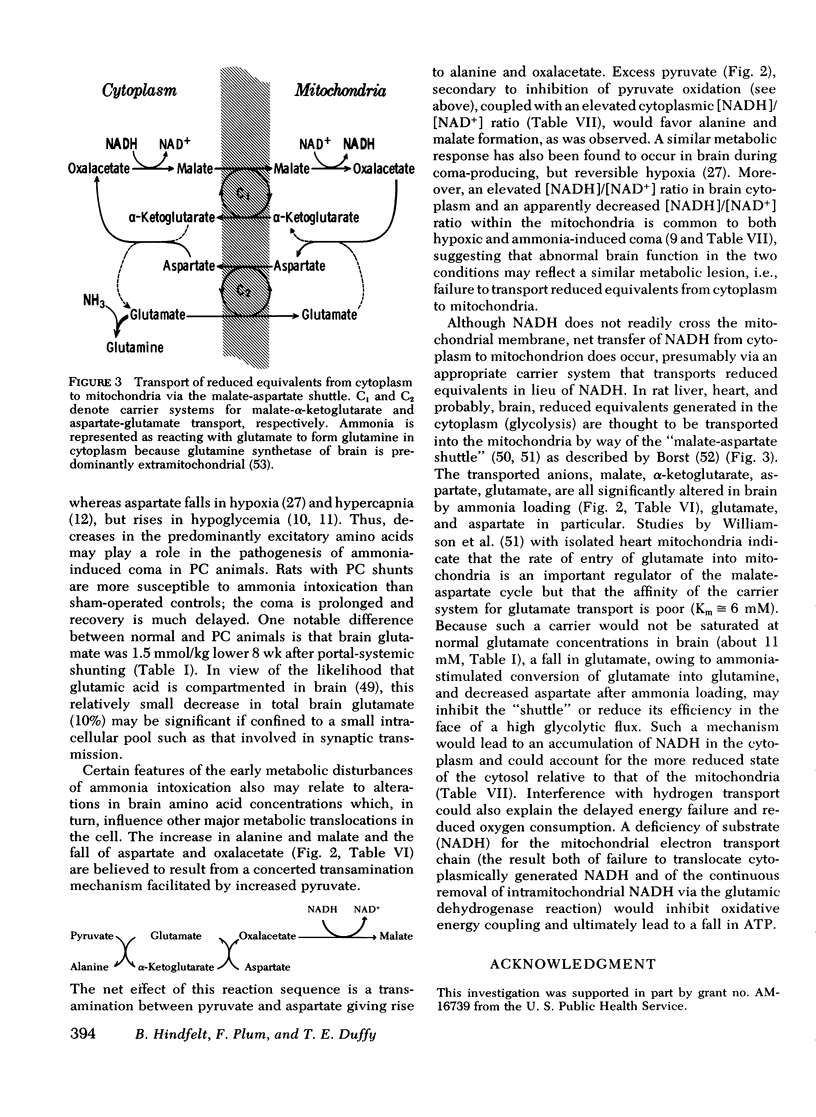
Rats were made chronically hyperammonemic by portal-systemic shunting and, 8 wk later, were subjected to acute ammonia intoxication by the intraperitoneal injection of 5.2 mmol/kg of ammonium acetate. In free-ranging animals, ammonia treatment induced a brief period of precoma (10-15 min) that progressed into deep, anesthetic coma lasting for several hours and was associated with a high mortality. In paralyzed, artificially ventilated animals that were lightly anesthetized with nitrous oxide, acute ammonia intoxication caused major disturbances of cerebral carbohydrate, amino acid, and energy metabolism that correlated in time with the change in functional state. At 10 min after injection (precoma), the concentrations of most glycolytic intermediates were increased, as was the lactate/pyruvate ratio. Citrate declined, despite a twofold rise in pyruvate, suggesting that the conversion of pyruvate to citrate had been impaired. Concentrations of phosphocreatine, and of the putative neurotransmitters, glutamate and aspartate, declined during precoma, but the concentrations of the adenine nucleotides in the cerebral hemispheres, cerebellum, and brain stem remained within normal limits. At 60 min after injection (coma), ATP declined in all regions of brain; the reduction in total high-energy phosphates was most notable in the brain stem. The findings indicate that cerebral dysfunction in chronic, relapsing ammonia intoxication is not due to primary energy failure. Rather, it is suggested that ammonia-induced depletion of glutamic and aspartic acids, and inhibition of the malate-asparate hydrogen shuttle are the dominant neurochemical lesions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berl S. Cerebral amino acid metabolism in hepatic coma. Exp Biol Med. 1971;4:71–84. [PubMed] [Google Scholar]
- Breen K. J., Schenker S. Hepatic coma: present concepts of pathogenesis and therapy. Prog Liver Dis. 1972;4:301–332. [PubMed] [Google Scholar]
- Cavanagh J. B. Liver bypass and the glia. Res Publ Assoc Res Nerv Ment Dis. 1974;53:13–38. [PubMed] [Google Scholar]
- Duffy T. E., Nelson S. R., Lowry O. H. Cerebral carbohydrate metabolism during acute hypoxia and recovery. J Neurochem. 1972 Apr;19(4):959–977. doi: 10.1111/j.1471-4159.1972.tb01417.x. [DOI] [PubMed] [Google Scholar]
- Folbergrová J., MacMillan V., Siesjö B. K. The effect of hypercapnic acidosis upon some glycolytic and Krebs cycle-associated intermediates in the rat brain. J Neurochem. 1972 Nov;19(11):2507–2517. doi: 10.1111/j.1471-4159.1972.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Folbergrová J., Passonneau J. V., Lowry O. H., Schulz D. W. Glycogen, ammonia and related metabolities in the brain during seizures evoked by methionine sulphoximine. J Neurochem. 1969 Feb;16(2):191–203. doi: 10.1111/j.1471-4159.1969.tb05937.x. [DOI] [PubMed] [Google Scholar]
- Gjedde A., Lockwood A., Duffy T. E., Plum F. Effect of ammonia on cerebral metabolism of rats with portocaval shunts. Trans Am Neurol Assoc. 1976;101:180–181. [PubMed] [Google Scholar]
- Hawkins R. A., Miller A. L., Nielsen R. C., Veech R. L. The acute action of ammonia on rat brain metabolism in vivo. Biochem J. 1973 Aug;134(4):1001–1008. doi: 10.1042/bj1341001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindfelt B., Siesjö B. K. Cerebral effects of acute ammonia intoxication. I. The influence on intracellular and extracellular acid-base parameters. Scand J Clin Lab Invest. 1971 Nov;28(3):353–364. doi: 10.3109/00365517109095710. [DOI] [PubMed] [Google Scholar]
- Hindfelt B., Siesjö B. K. Cerebral effects of acute ammonia intoxication. II. The effect upon energy metabolism. Scand J Clin Lab Invest. 1971 Nov;28(3):365–374. doi: 10.3109/00365517109095711. [DOI] [PubMed] [Google Scholar]
- Hindfelt B. The effect of sustained hyperammonemia upon the metabolic state of the brain. Scand J Clin Lab Invest. 1972 Nov;30(3):245–255. doi: 10.3109/00365517209084286. [DOI] [PubMed] [Google Scholar]
- Holmin T., Siesjö B. K. The effect of porta-caval anastomosis upon the energy state and upon acid-base parameters of the rat brain. J Neurochem. 1974 Mar;22(3):403–412. doi: 10.1111/j.1471-4159.1974.tb07606.x. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B., SCOTT E. M. Aldehyde oxidation. III. Succinic semialdehyde dehydrogenase. J Biol Chem. 1959 Apr;234(4):937–940. [PubMed] [Google Scholar]
- James I. M., MacDonnell L., Xanalatos C. Effect of ammonium salts on brain metabolism. J Neurol Neurosurg Psychiatry. 1974 Aug;37(8):948–953. doi: 10.1136/jnnp.37.8.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi H., Shore N. S., Shih V. E., Shannon D. C. Brain organic buffers in respiratory acidosis and alkalosis. J Appl Physiol. 1973 Apr;34(4):478–482. doi: 10.1152/jappl.1973.34.4.478. [DOI] [PubMed] [Google Scholar]
- Kyu M. H., Cavanagh J. B. Some effects of porto-caval anastomosis in the male rat. Br J Exp Pathol. 1970 Apr;51(2):217–227. [PMC free article] [PubMed] [Google Scholar]
- LASSEN N. A. Cerebral blood flow and oxygen consumption in man. Physiol Rev. 1959 Apr;39(2):183–238. doi: 10.1152/physrev.1959.39.2.183. [DOI] [PubMed] [Google Scholar]
- LEE S. H., FISHER B. Portacaval shunt in the rat. Surgery. 1961 Oct;50:668–672. [PubMed] [Google Scholar]
- Lewis L. D., Ljunggren B., Norberg K., Siesjö B. K. Changes in carbohydrate substrates, amino acids and ammonia in the brain during insulin-induced hypoglycemia. J Neurochem. 1974 Oct;23(4):659–671. doi: 10.1111/j.1471-4159.1974.tb04389.x. [DOI] [PubMed] [Google Scholar]
- Ljunggren B., Schutz H., Siesjö B. K. Changes in energy state and acid-base parameters of the rat brain during complete compression ischemia. Brain Res. 1974 Jun 20;73(2):277–289. doi: 10.1016/0006-8993(74)91049-x. [DOI] [PubMed] [Google Scholar]
- MANN J. D., BOLLMAN J. L., HUIZENGA K. A., FARRAR T., GRINDLAY J. H. Blood ammonia, experimental and clinical study in abnormalities of the liver and portal circulation. Gastroenterology. 1954 Oct;27(4):399–410. [PubMed] [Google Scholar]
- MCKHANN G. M., TOWER D. B. Ammonia toxicity and cerebral oxidative metabolism. Am J Physiol. 1961 Mar;200:420–424. doi: 10.1152/ajplegacy.1961.200.3.420. [DOI] [PubMed] [Google Scholar]
- MEISTER A. Studies on the mechanism and specificity of the glutamine-alpha-keto acid transamination-deamidation reaction. J Biol Chem. 1954 Sep;210(1):17–35. [PubMed] [Google Scholar]
- McDERMOTT W. V., Jr, ADAMS R. D. Episodic stupor associated with an Eck fistula in the human with particular reference to the metabolism of ammonia. J Clin Invest. 1954 Jan;33(1):1–9. doi: 10.1172/JCI102862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. L., Hawkins R. A., Veech R. L. The mitochondrial redox state of rat brain. J Neurochem. 1973 May;20(5):1393–1400. doi: 10.1111/j.1471-4159.1973.tb00251.x. [DOI] [PubMed] [Google Scholar]
- Nelson S. R., Schulz D. W., Passonneau J. V., Lowry O. H. Control of glycogen levels in brain. J Neurochem. 1968 Nov;15(11):1271–1279. doi: 10.1111/j.1471-4159.1968.tb05904.x. [DOI] [PubMed] [Google Scholar]
- PHILLIPS G. B., SCHWARTZ R., GABUZDA G. J., Jr, DAVIDSON C. S. The syndrome of impending hepatic coma in patients with cirrhosis of the liver given certain nitrogenous substances. N Engl J Med. 1952 Aug 14;247(7):239–246. doi: 10.1056/NEJM195208142470703. [DOI] [PubMed] [Google Scholar]
- Plum F. The CSF in hepatic encephalopathy. Exp Biol Med. 1971;4:34–41. [PubMed] [Google Scholar]
- SELLINGER O. Z., VERSTER F. D. Glutamine synthetase of rat cerebral cortex: intracellular distribution and structural latency. J Biol Chem. 1962 Sep;237:2836–2844. [PubMed] [Google Scholar]
- Schenker S., McCandless D. W., Brophy E., Lewis M. S. Studies on the intracerebral toxicity of ammonia. J Clin Invest. 1967 May;46(5):838–848. doi: 10.1172/JCI105583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. K., Heine J. D. Effect of insulin on nitrogenous constituents of rat brain. J Neurochem. 1965 Jun;12(6):527–532. doi: 10.1111/j.1471-4159.1965.tb06780.x. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Folbergrová J., MacMillan V. The effect of hypercapnia upon intracellular pH in the brain, evaluated by the bicarbonate-carbonic acid method and from the creatine phosphokinase equilibrium. J Neurochem. 1972 Nov;19(11):2483–2495. doi: 10.1111/j.1471-4159.1972.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Nilsson L. The influence of arterial hypoxemia upon labile phosphates and upon extracellular and intracellular lactate and pyruvate concentrations in the rat brain. Scand J Clin Lab Invest. 1971 Feb;27(1):83–96. doi: 10.3109/00365517109080193. [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Boer K. The presence of biologically labile compounds during ischemia and their relationship to the EEG in rat cerebral cortex and hypothalamus. J Neurochem. 1972 Dec;19(12):2843–2853. doi: 10.1111/j.1471-4159.1972.tb03822.x. [DOI] [PubMed] [Google Scholar]
- THORN W., HEIMANN J. Beeinflussung der Ammoniak-Konzentration in Gehirn, Herz, Leber, Niere und Muskulatur durch Ischämie, Anoxie, Asphyxie und Hypothermie. J Neurochem. 1958;2(2-3):166–177. doi: 10.1111/j.1471-4159.1958.tb12361.x. [DOI] [PubMed] [Google Scholar]
- Tews J. K., Carter S. H., Stone W. E. Chemical changes in the brain during insulin hypoglycaemia and recovery. J Neurochem. 1965 Aug;12(8):679–693. doi: 10.1111/j.1471-4159.1965.tb06782.x. [DOI] [PubMed] [Google Scholar]
- Tews J. K., Stone W. E. Free amino acids and related compounds in brain and other tissues: effects of convulsant drugs. Prog Brain Res. 1965;16:135–163. doi: 10.1016/s0079-6123(08)61398-9. [DOI] [PubMed] [Google Scholar]
- Vannucci R. C., Duffy T. E. Influence of birth on carbohydrate and energy metabolism in rat brain. Am J Physiol. 1974 Apr;226(4):933–940. doi: 10.1152/ajplegacy.1974.226.4.933. [DOI] [PubMed] [Google Scholar]
- Vergara F., Plum F., Duffy T. E. Alpha-ketoglutaramate: increased concentrations in the cerebrospinal fluid of patients in hepatic coma. Science. 1974 Jan 11;183(4120):81–83. doi: 10.1126/science.183.4120.81. [DOI] [PubMed] [Google Scholar]
- Victor M., Adams R. D., Cole M. The acquired (non-Wilsonian) type of chronic hepatocerebral degeneration. Medicine (Baltimore) 1965 Sep;44(5):345–396. doi: 10.1097/00005792-196509000-00001. [DOI] [PubMed] [Google Scholar]
- WORCEL A., ERECINSKA M. Mechanism of inhibitory action of ammonia on the respiration of rat-liver mitochondria. Biochim Biophys Acta. 1962 Nov 19;65:27–33. doi: 10.1016/0006-3002(62)90146-4. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Jakob A., Refino C. Control of the removal of reducing equivalents from the cytosol in perfused rat liver. J Biol Chem. 1971 Dec 25;246(24):7632–7641. [PubMed] [Google Scholar]



