Abstract
Purpose
This study assessed changes in functional dysmetria (FD) and in brain activation observable by functional magnetic resonance imaging (fMRI) during a leg flexion-extension motor task following brain stimulation with a single radioelectric asymmetric conveyer (REAC) pulse, according to the precisely defined neuropostural optimization (NPO) protocol.
Population and methods
Ten healthy volunteers were assessed using fMRI conducted during a simple motor task before and immediately after delivery of a single REAC-NPO pulse. The motor task consisted of a flexion-extension movement of the legs with the knees bent. FD signs and brain activation patterns were compared before and after REAC-NPO.
Results
A single 250-millisecond REAC-NPO treatment alleviated FD, as evidenced by patellar asymmetry during a sit-up motion, and modulated activity patterns in the brain, particularly in the cerebellum, during the performance of the motor task.
Conclusion
Activity in brain areas involved in motor control and coordination, including the cerebellum, is altered by administration of a REAC-NPO treatment and this effect is accompanied by an alleviation of FD.
Keywords: motor behavior, motor control, cerebellum, dysmetria, functional dysmetria, fluctuating asymmetry
Introduction
Many individuals, in the absence of organic injuries or orthopedic pathology, show a slight misalignment of body segments.1,2 This misalignment is associated with detectable asymmetries in the tonic and phasic activation of symmetrical muscular groups in the lower limbs that produce a stable misalignment of one’s left and right patellar margins1 during motions that are intended to be symmetrical, such as moving in a sit-up motion from a supine to a sitting position.2 This phenomenon, which we call functional dysmetria3 (FD), may be related to the effects of neuropsychomotor attitude on postural control and may be the consequence of an adaptation to environmental stress4–11 in animals12–14 as well as humans.15–19 As for finger-to-nose dysmetria, FD is difficult to evaluate as an absolute measurement.20 Therefore, the evaluation of FD must be made by medical experts and consists of observation of the presence or absence of FD. In assessing FD, a specific caliper specially designed for this type of evaluation21 (Dismetrometro, ASMED, Florence Italy) may be used. The radioelectric asymmetric conveyer REAC22,23 is an innovative medical device originally designed to use the effects produced by the interaction between the electromagnetic field of the human body and those produced by the instrument. REAC is an innovative technology for biostimulation and bioenhancement that uses weak radioelectric fields to modulate brain activity. REAC treatment has previously been reported to ameliorate several stress-related disorders,24–27 depression,26,28,29 anxiety,26,29 bipolar disorders,30 some forms of dementia,31 and impaired motor control.32,33 In a recent study, we examined the effects of REAC administered according to a precisely defined neuro-postural optimization3 (NPO) protocol on brain activation patterns during a finger tapping motor task. We found that changes in activity patterns in motor cerebral and cerebellar regions were evident following the REAC-NPO treatment.34
The aim of the present preliminary study was to examine whether the REAC-NPO treatment that was previously shown34 to affect brain activity patterns in motor regions during the aforementioned finger tapping task would affect FD evident during a lower limb motor task in which subjects are asked to perform a bilateral flexion-extension movement of the legs, bending the legs at the knees. Based on our clinical observations2,3 of improvements in balance and coordination following REAC-NPO in our patients, we hypothesized that the REAC-NPO treatment would reduce FD signs in the present experimental study group.
Material and methods
Study population
Our study was designed in accordance with the Helsinki Declaration. Each participant gave signed informed consent to participate in the study, which is registered in the Australian New Zealand Clinical Trial Registry (ACTRN12611000366954). Physically active, right-handed students who regularly engaged in running were recruited to ensure a cohort of subjects in good health, particularly with respect to motor performance ability. After screening ten volunteers for FD, a total of ten healthy graduate students (six females, four males), ranging in age from 25–32 years (mean, 28 years), were confirmed by two clinicians to show FD and volunteered to participate in this study without financial compensation. FD was analyzed by two expert clinicians as volunteers moved from a supine to a sitting position on a rigid surface. This action reveals the presence of FD as a misalignment of the patella (red line in Figure 1A).
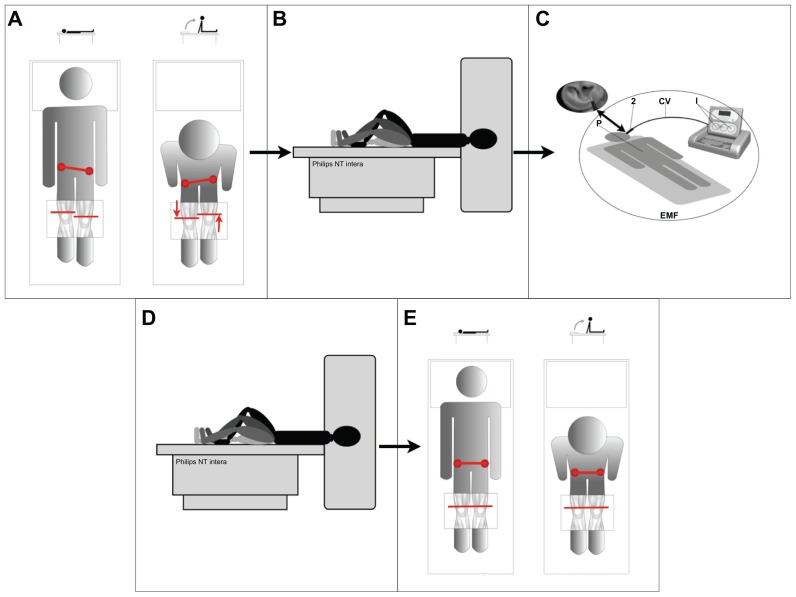
Figure 1.
Study design overview. (A) Initial assessment of functional dysmetria (FD). (B) Pre radio-electric asymmetric conveyer neuro-postural optimization (REAC-NPO) scans during performance of the motor task. (C) Administration of REAC-NPO. (D) Post REAC-NPO scans during performance of the motor task. (E) Post-REAC-NPO assessment of FD.
Study design
As summarized in Figure 1, the volunteers were subjected to two FD assessments and two functional magnetic resonance imaging (fMRI) scans. Before REAC-NPO, each subject was assessed for FD as described above (Figure 1A) and then scanned while performing the alternating leg flexion-extension motor task described below (Figure 1B). Immediately following the scan, the subject was brought to a designated treatment room for delivery of the REAC pulse (Figure 1C). Forty minutes after the REAC-NPO, the subject was brought back to the magnet room and scanned while performing the alternating leg flexion-extension motor task, as described below (Figure 1D). Following the second scan, a post REAC-NPO assessment was performed (Figure 1E).
Motor task
The motor task consisted of a simple “block” of leg-bending movements with alternating flexion and extension of the knee with the subject lying in a supine position (Figure 1B). Within each block, there was 30 seconds of cyclical movement followed by a 30-second rest period. Each subject performed 30 blocks of the motor task during each scan.
REAC-NPO
A Convogliatore di Radianza Modulante REAC instrument (ASMED, Florence, Italy) specific for noninvasive brain stimulation techniques was used in this study. The REAC-NPO protocol used consisted of a single 250-millisecond radiofrequency burst administered at 5.8 GHz. The REAC probe is placed at the level of the scapha28,32 when the radiofrequency pulse is delivered (Figure 1C). The REAC procedure is painless, and no adverse effects from its use have been reported. The density of the radio-electric current flowing to the subject (150 cm from the emitter) during the single radiofrequency burst is 7 μA/cm2 and the electromagnetic field surrounding the device is approximately 20 μW/m2.
Image acquisition
Brain fMRI was performed with a high-field unit (1.5 T Philips Intera NT, The Netherlands), shown in Figure 1B. The survey was obtained with sequences of centering axial, sagittal, and coronal planes. Volumetric sequences (with gradient echo T1 (3D TFE; TR = l3, TE = 3, FA = 30) and T2 (EPI-FFE; TR = 3000, TE = 50, FA = 90)) were oriented upon these planes. The total duration of the fMRI acquisition, conducted while the subject performed the alternating leg flexion-extension motor task, was about 40 minutes. Two radiologists who were not present for the REAC-NPO treatments performed the scans.
Image processing
Acquired DICOM images were sent to a computer running on a LINUX operating system and then exported as com pressed NIFTI files. The NIFTI files were then processed using FEAT (fMRI Expert Analysis Tool) software in the FMRIB software library (FSL). Two radiologists performed the first-level statistical analysis concurrently. Brain tissue was isolated from surrounding tissues using the Brain Extraction Tool (BET) in the FSL. The MCFLIRT Motion Correction tool was used to correct for misalignment due to subjects’ inadvertent movements using an algorithm based on the FLIRT technique of intra- and intermodal brain image registration. The output was then processed with FMRIB’s Improved Linear Modeling (FILM) tool in accordance with the block diagram of the study and taking into account the spatial parameters of the head motion detected by the MCFLIRT tool. A high-pass filter was used to remove low-frequency artifacts. The results were coregistered (ie, the different sessions were registered to each other with FLIRT).
Statistical analysis
A t-test was performed for the statistical analysis. The relative Z statistic image was designated with a Z-threshold of 4.6 to show which clusters were activated significantly (P = 0.05). The activation images were rendered with a pseudocolor scale (Z value range, 4.6–9) and then superimposed onto a standard brain template. To assess inter-subject and inter-group variance and average activation within each phase of the experiment (before and after REAC-NPO), the output of the first-level statistical analysis was processed using FMRIB’s Local Analysis of Mixed Effects algorithm, which eliminates outlier data. An arbitrary Z-threshold of 2.3 was set to enable rendering in a pseudocolor scale using a contiguous-clusters technique (significance level P = 0.05). Activation images were superimposed onto MN 1–152 standard brain atlas template images.
Results
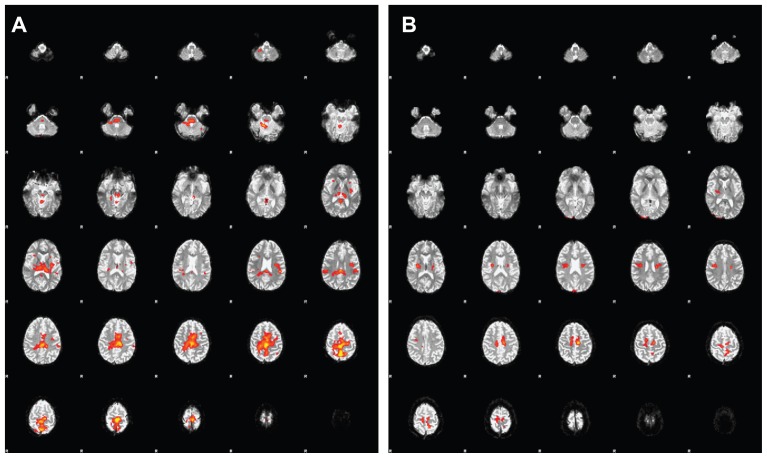
The FD (evidenced by misalignment of the patellae during a sit-up movement) observed before REAC-NPO treatment was alleviated in all ten subjects at the time of the second, post REAC-NPO fMRI scan. That is, two expert clinicians reported that the subjects showed proper alignment of the patellae at the second scan (see Figure 1E). Due to the presence of artifacts that degraded the functional images, one subject was excluded from our second-level statistical analysis. The averaged fMRI datasets from the remaining nine subjects before and after REAC-NPO are illustrated in Figure 2. In particular, note that there was markedly less activation in motor cortical areas, disappearance of right thalamic activation, and reduced activation in the cerebellar vermis and pontomesencephalic regions in the post REAC-NPO averaged images (Figure 2A) relative to the pre REAC-NPO averaged images (Figure 2B).
Figure 2.
Average of functional magnetic resonance imaging (fMRI) scans from nine subjects before (A) and after (B) radioelectric asymmetric conveyer neuro-postural optimization (REAC-NPO).
Discussion
The current study showed that a 250-millisecond REAC-NPO pulse was able to produce changes in brain activity that were apparent 40 minutes after delivery of the REAC stimulation. This observation implies there are stable changes in brain activation patterns, including activation of brain areas involved in motor control. Additionally, following the REAC-NPO treatment, we observed improved patellar symmetry when subjects were asked to perform a sit-up motion, indicating they experienced a concomitant amelioration of FD. We suggest that the presently observed reduction in FD may be due to brain activity changes enabling a more efficient motor control and motor strategy,35 similar to that described following other brain stimulation techniques.36 The modulation of cerebellar function is the most important finding of this study, considering the importance of the cerebellum in motor control.37–40 Moreover, there is growing evidence implicating the cerebellum in stress-influenced affective41 and cognitive42–44 functions.
Interestingly, numerous studies have related stress to various forms of dysmetria, including motor dysmetria and behavioral and cognitive dysmetria phenomena such as cognitive dysmetria,45,46 dysmetria of thought,47–50 and cerebellar cognitive affective syndrome.48,51–53 These forms of dysmetria do not appear to result from any anatomical or physiological lesion, but rather seem to reflect a cerebellar dysfunction.47,50 Hence, FD may be the result of dysfunctional adaptive phenomena that involve the complex interrelationships between cerebellar, cerebral motor, cognitive, emotional, and behavioral functions. Since in our clinical experience the positive effects of REAC-NPO on coordination and FD tend to persist for long periods of time, even years,3 we have an interest in examining the potential of REAC-NPO for remodeling cerebellar activation related to coordination and precision of movement. Additional research is needed to delineate the physiological mechanisms underlying the establishment and alleviation of FD. Furthermore, longer-term controlled studies are needed to elucidate the long-term stability of changes in the brain activation pattern and FD alleviation observed following REAC-NPO.
Conclusions
REAC-NPO may be useful to correct FD. Correction of FD may be particularly helpful in the rehabilitation of patients showing FD in addition to a motor physiological pathology, as improved coordination may facilitate recovery. Additional studies to compare the brain activities of an experimental stimulation group with those of a sham stimulation group are needed.
Acknowledgments
We would like to thank the technical staff of Diagnostic Imaging Service at Azienda Ospedaliero Universitaria in Cagliari for their assistance and Dr Francesco Sias, MD for his collaboration. The Italian Society of Neuro Psycho Physical Optimization financially supported this study.
Footnotes
Disclosure
Salvatore Rinaldi and Vania Fontani are the inventors of the Radio-Electric Asymmetric Conveyer.
References
- 1.Siqueira CM, Lahoz Moya GB, Caffaro RR, et al. Misalignment of the knees: Does it affect human stance stability. J Bodyw Mov Ther. 2011;15(2):235–241. doi: 10.1016/j.jbmt.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Evaluation of dysfunctional patellar misalignment at the passage from the supine to the sitting position and vice versa in patients with a negative anamnesis for muscular or bone-relevant trauma. Australian New Zealand Clinical Trials Register. 2007. Available from: http://www.anzctr.org.au/trial_view.aspx?id=82480.
- 3.Evaluation of the effectiveness of the Neuro Postural Optimization therapy with conveyer of modulating radiance to treat functional dysmetria. Australian New Zealand Clinical Trials Registry. 2008. Available from: http://www.anzctr.org.au/trial_view.aspx?id=82524.
- 4.Schell LM, Johnston FE, Smith DR, Paolone AM. Directional asymmetry of body dimensions among white adolescents. Am J Phys Anthropol. 1985 Aug;67(4):317–322. doi: 10.1002/ajpa.1330670404. [DOI] [PubMed] [Google Scholar]
- 5.Deleon VB. Fluctuating asymmetry and stress in a medieval Nubian population. Am J Phys Anthropol. 2007 Apr;132(4):520–534. doi: 10.1002/ajpa.20549. [DOI] [PubMed] [Google Scholar]
- 6.Knierim U, Van Dongen S, Forkman B, et al. Fluctuating asymmetry as an animal welfare indicator – a review of methodology and validity. Physiol Behav. 2007 Oct 22;92(3):398–421. doi: 10.1016/j.physbeh.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Parsons PA. Fluctuating asymmetry: an epigenetic measure of stress. Biol Rev Camb Philos Soc. 1990 May;65(2):131–145. doi: 10.1111/j.1469-185x.1990.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 8.Parsons PA. Fluctuating asymmetry and stress intensity. Trends Ecol Evol. 1990 Mar;5(3):97–98. doi: 10.1016/0169-5347(90)90240-E. [DOI] [PubMed] [Google Scholar]
- 9.Leary RF, Allendorf FW. Fluctuating asymmetry as an indicator of stress: Implications for conservation biology. Trends Ecol Evol. 1989 Jul;4(7):214–217. doi: 10.1016/0169-5347(89)90077-3. [DOI] [PubMed] [Google Scholar]
- 10.Newell-Morris LL, Fahrenbruch CE, Sackett GP. Prenatal psychological stress, dermatoglyphic asymmetry and pregnancy outcome in the pigtailed macaque (Macaca nemestrina) Biol Neonate. 1989;56(2):61–75. doi: 10.1159/000243104. [DOI] [PubMed] [Google Scholar]
- 11.Ovchinnikov ND. Changes in the interhemispheric functional asymmetry of the brain and parameters of professional reliability of operators in the process of work under high neuro-emotional stress. Fiziol Cheloveka. 1998 Mar-Apr;24(2):74–79. [PubMed] [Google Scholar]
- 12.Hoffmann KP, Bremmer F, Thiele A, Distler C. Directional asymmetry of neurons in cortical areas MT and MST projecting to the NOT-DTN in macaques. J Neurophysiol. 2002 Apr;87(4):2113–2123. doi: 10.1152/jn.00488.2001. [DOI] [PubMed] [Google Scholar]
- 13.Alonso SJ, Navarro E, Santana C, Rodriguez M. Motor lateralization, behavioral despair and dopaminergic brain asymmetry after prenatal stress. Pharmacol Biochem Behav. 1997 Oct;58(2):443–448. doi: 10.1016/s0091-3057(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 14.McKenzie JA. Stress and asymmetry during arrested development of the Australian sheep blowfly. Proc Biol Sci. 1997 Dec 22;264(1389):1749–1756. doi: 10.1098/rspb.1997.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozener B. Fluctuating and directional asymmetry in young human males: effect of heavy working condition and socioeconomic status. Am J Phys Anthropol. 2010 Sep;143(1):112–120. doi: 10.1002/ajpa.21300. [DOI] [PubMed] [Google Scholar]
- 16.Lazenby RA, Cooper DM, Angus S, Hallgrimsson B. Articular constraint, handedness, and directional asymmetry in the human second metacarpal. J Hum Evol. 2008 Jun;54(6):875–885. doi: 10.1016/j.jhevol.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Monk TH, Buysse DJ, Carrier J, Kupfer DJ. Inducing jet-lag in older people: directional asymmetry. J Sleep Res. 2000 Jun;9(2):101–116. doi: 10.1046/j.1365-2869.2000.00184.x. [DOI] [PubMed] [Google Scholar]
- 18.Swinnen SP, Vangheluwe S, Wagemans J, et al. Shared neural resources between left and right interlimb coordination skills: the neural substrate of abstract motor representations. Neuroimage. 2010 Feb 1;49(3):2570–2580. doi: 10.1016/j.neuroimage.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 19.Carrion VG, Weems CF, Eliez S, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001 Dec 15;50(12):943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 20.Swaine BR, Sullivan SJ. Reliability of the scores for the finger-to- nose test in adults with traumatic brain injury. Phys Ther. 1993 Feb;73(2):71–78. doi: 10.1093/ptj/73.2.71. [DOI] [PubMed] [Google Scholar]
- 21.A new patellar misalignment measurement device: Dismetrometro. Australian New Zealand Clinical Trials Registry. 2007. Available from: http://www.anzctr.org.au/trial_view.aspx?id=82346.
- 22.Rinaldi S, Fontani V, assignees; Rinaldi S, Fontani V, inventors. Patent EP1301241 (B1) Radioelectric Asymmetric Conveyer for therapeutic use. 2006 Oct 11; 2000.
- 23.Rinaldi S, Fontani V, inventors; Rinaldi S, Fontani V, assignees. US patent 7,333859. Radioelectric Asymmetric Conveyer for therapeutic use. 2008 Feb 19; 2001.
- 24.Collodel G, Moretti E, Fontani V, et al. Effect of emotional stress on sperm quality. Indian J Med Res. 2008 Sep;128(3):254–261. [PubMed] [Google Scholar]
- 25.Rinaldi S, Fontani V, Aravagli L, Margotti ML. Psychological and symptomatic stress-related disorders with radio-electric treatment: Psychometric evaluation. Stress Health. 2010;26(5):350–358. [Google Scholar]
- 26.Rinaldi S, Fontani V, Aravagli L, Mannu P. Psychometric evaluation of a radio electric auricular treatment for stress related disorders: a double-blinded, placebo-controlled controlled pilot study. Health Qual Life Outcomes. 2010 Mar 20;8(1):31. doi: 10.1186/1477-7525-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mannu P, Rinaldi S, Fontani V, Castagna A. Radio electric asymmetric brain stimulation in the treatment of behavioral and psychiatric symptoms in Alzheimer disease. Clin Interv Aging. 2011;6:207–211. doi: 10.2147/CIA.S23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mannu P, Rinaldi S, Fontani V, Castagna A, Lotti Margotti M. Radio electric treatment vs Es-Citalopram in the treatment of panic disorders associated with major depression: An open-label, naturalistic study. Acupuncture and Electro – Therapeutics Res Int J. 2009;34:135–149. doi: 10.3727/036012909803861040. [DOI] [PubMed] [Google Scholar]
- 29.Olivieri EB, Vecchiato C, Ignaccolo N, et al. Radioelectric brain stimulation in the treatment of generalized anxiety disorder with comorbid major depression in a psychiatric hospital: a pilot study. Neuropsychiatr Dis Treat. 2011;7:449–455. doi: 10.2147/NDT.S23420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannu P, Rinaldi S, Fontani V, Castagna A. Long-term treatment of bipolar disorder with a radioelectric asymmetric conveyor. Neuropsychiatr Dis Treat. 2011;7:373–379. doi: 10.2147/NDT.S22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontani V, Rinaldi S, Aravagli L, Mannu P, Castagna A, Margotti ML. Noninvasive radioelectric asymmetric brain stimulation in the treatment of stress-related pain and physical problems: psychometric evaluation in a randomized, single-blind placebo-controlled, naturalistic study. Int J Gen Med. 2011 Sep 22;4(1):681–686. doi: 10.2147/IJGM.S24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castagna A, Rinaldi S, Fontani V, Aravagli L, Mannu P, Margotti ML. Does osteoarthritis of the knee also have a psychogenic component? Psycho-emotional treatment with a radio-electric device vs intra- articular injection of sodium hyaluronate: An open-label, naturalistic study. Acupuncture and Electro – Therapeutics Res Int J. 2010;35(1–2):1–16. doi: 10.3727/036012910803860968. [DOI] [PubMed] [Google Scholar]
- 33.Castagna A, Rinaldi S, Fontani V, Mannu P, Margotti ML. Radio electric asymmetric brain stimulation and lingual apex repositioning in patients with atypical deglutition: a naturalistic, open-label study. J Multidiscip Healthc. 2011 doi: 10.2147/JMDH.S22830. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaldi S, Fontani V, Castagna A. Brain activity modif ication produced by a single radio electric asymmetric brain stimulation pulse: a new tool for neuropsychiatric treatments. Preliminary fMRI study. Neuropsychiatr Dis Treat. 2011 Oct 28;7(1):649–654. doi: 10.2147/NDT.S26123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penhune VB, Zattore RJ, Evans AC. Cerebellar contributions to motor timing: a PET study of auditory and visual rhythm reproduction. J Cogn Neurosci. 1998 Nov;10(6):752–765. doi: 10.1162/089892998563149. [DOI] [PubMed] [Google Scholar]
- 36.Zimerman M, Hummel FC. Non-invasive brain stimulation: enhancing motor and cognitive functions in healthy old subjects. Front Aging Neurosci. 2010;2:149. doi: 10.3389/fnagi.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manto M, Bower JM, Conforto AB, et al. Consensus paper: Roles of the cerebellum in motor control-the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2011 Dec 13; doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010 Jul-Aug;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terao Y. Studying adaptive motor control of the cerebellum by the precision grip paradigm. Clin Neurophysiol. 2008 Nov;119(11):2419–2420. doi: 10.1016/j.clinph.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Hirano T. Motor control mechanism by the cerebellum. Cerebellum. 2006;5(4):296–300. doi: 10.1080/14734220600776387. [DOI] [PubMed] [Google Scholar]
- 41.Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006 Feb;129(Pt 2):290–292. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- 42.Timmann D, Daum I. Cerebellar contributions to cognitive functions: a progress report after two decades of research. Cerebellum. 2007;6(3):159–162. doi: 10.1080/14734220701496448. [DOI] [PubMed] [Google Scholar]
- 43.Thach WT. On the mechanism of cerebellar contributions to cognition. Cerebellum. 2007;6(3):163–167. doi: 10.1080/14734220701373530. [DOI] [PubMed] [Google Scholar]
- 44.Della Sala S. Cognition and the cerebellum. Cortex. 2011 Jan;47(1):1. doi: 10.1016/j.cortex.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999 Oct 1;46(7):908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- 46.Friston K. Disconnection and cognitive dysmetria in schizophrenia. Am J Psychiatry. 2005 Mar;162(3):429–432. doi: 10.1176/appi.ajp.162.3.429. [DOI] [PubMed] [Google Scholar]
- 47.Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010 Sep;20(3):236–260. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- 48.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. Summer. 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 49.Schmahmann JD. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci. 1998 Sep 1;2(9):362–371. doi: 10.1016/s1364-6613(98)01218-2. [DOI] [PubMed] [Google Scholar]
- 50.Andreasen NC, O’Leary DS, Cizadlo T, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Partridge J, Rayner J, Awan S. The cerebellar cognitive affective syndrome. Br J Hosp Med (Lond) 2010 Dec;71(12):712–713. doi: 10.12968/hmed.2010.71.12.712. [DOI] [PubMed] [Google Scholar]
- 52.Wolf U, Rapoport MJ, Schweizer TA. Evaluating the affective component of the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. Summer. 2009;21(3):245–253. doi: 10.1176/jnp.2009.21.3.245. [DOI] [PubMed] [Google Scholar]
- 53.Manto M. Mechanisms of human cerebellar dysmetria: experimental evidence and current conceptual bases. J Neuroeng Rehabil. 2009;6:10. doi: 10.1186/1743-0003-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]