Abstract
This study reports the development of a specific and sensitive radioimmunoassay and a simple and accurate radial immunodiffusion (RID) assay for the human serum-binding protein for vitamin D and its metabolites (DBP). These immunoassays employed a monospecific antiserum that was prepared in rabbits against human DBP. The radioimmunoassay effectively measured DBP in amounts of 1-10 ng, whereas the RID assay measured DBP accurately in amounts of 0.2-0.8 mug. The results obtained with the two immunoassays on the same samples of serum agreed well with each other. Using the RID assay, the mean (+/- SD) serum DBP concentration observed in 35 normal persons was 422 +/- 27 micrograms/ml. Generally similar levels were observed in 66 hyperlipidemic subjects. In molar terms, the mean DBP concentration (approximately 8 microgramsM) was of the order of 50 times the usual serum level of 25-hydroxyvitamin D (25-OH-D) plus vitamin D. Thus, most of plasma DBP circulates as apo-DBP, not containing a bound molecule of 25-OH-D or of vitamin D. DBP and 25-OH-D concentrations were measured in a limited number of patients with hypercalcemia, mild hypocalcemia, and markedly elevated serum 25-OH-D levels due to oral vitamin D supplementation. It was found that major changes can occur in the serum levels of 25-OH-D and of calcium with very little or no associated changes occurring in the serum concentration of DBP, The results suggest that neither serum 25-OH-D nor serum calcium plays an important role in the regulation of the metabolism of DBP. Data were obtained that confirmed and extended an earlier report on the identity of the group-specific component (Gc) protein in plasma with the plasma vitamin D-binding protein. On immunodiffusion against whole serum, the line formed with the anti-DBP antiserum showed a complete reaction-of-identity with the line formed with commercial antiserum against Gc protein. Furthermore, serum that had been depleted of DBP by treatment with Sepharose containing covalently coupled antibodies against DBP was found to be depleted also of immunoreactivity against anti-GC protein antiserum. In addition, the properties of the purified DBP preparation agreed closely with those previously reported by others for Gc protein. Finally, a comparative immunology study showed that sera from several different mammalian orders showed some immunoreactivity against the antihuman DBP antiserum. Thus, proteins immunologically similar to human DBP are present in sera from a number of mammalian species and orders.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEARN A. G., KITCHIN F. D., BOWMAN B. H. HETEROGENEITY OF THE INHERITED GROUP-SPECIFIC COMPONENT OF HUMAN SERUM. J Exp Med. 1964 Jul 1;120:83–91. doi: 10.1084/jem.120.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayard F., Bec P., Louvet J. P. Measurement of plasma 25-hydroxycholecalciferol in man. Eur J Clin Invest. 1972 Jun;2(4):195–198. doi: 10.1111/j.1365-2362.1972.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Belsey R. E., DeLuca H. F., Potts J. T., Jr A rapid assay for 25-OH-vitamin D3 without preparative chromatography. J Clin Endocrinol Metab. 1974 Jun;38(6):1046–1051. doi: 10.1210/jcem-38-6-1046. [DOI] [PubMed] [Google Scholar]
- Bouillon R., Kerkhove P. V., De Moor P. Measurement of 25-hydroxyvitamin D3 in serum. Clin Chem. 1976 Mar;22(3):364–368. [PubMed] [Google Scholar]
- Bouillon R., Reynaert J., Claes J. H., Lissens W., De Moor P. The effect of anticonvulsant therapy on serum levels of 25-hydroxy-vitamin D, calcium, and parathyroid hormone. J Clin Endocrinol Metab. 1975 Dec;41(06):1130–1135. doi: 10.1210/jcem-41-6-1130. [DOI] [PubMed] [Google Scholar]
- Bouillon R., Van Baelen H., Rombauts W., De Moor P. The purification and characterisation of the human-serum binding protein for the 25-hydroxycholecalciferol (transcalciferin). Identity with group-specific component. Eur J Biochem. 1976 Jul 1;66(2):285–291. doi: 10.1111/j.1432-1033.1976.tb10518.x. [DOI] [PubMed] [Google Scholar]
- CLEVE H., PRUNIER J. H., BEARN A. G. ISOLATION AND PARTIAL CHARACTERIZATION OF THE TWO PRINCIPAL INHERITED GROUP-SPECIFIC COMPONENTS OF HUMAN SERUM. J Exp Med. 1963 Nov 1;118:711–726. doi: 10.1084/jem.118.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleve H. The variants of the group-specific component. A review of their distribution in human populations. Isr J Med Sci. 1973 Sep-Oct;9(9):1133–1146. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Daiger S. P., Schanfield M. S., Cavalli-Sforza L. L. Group-specific component (Gc) proteins bind vitamin D and 25-hydroxyvitamin D. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2076–2080. doi: 10.1073/pnas.72.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Edelstein S., Charman M., Lawson D. E., Kodicek E. Competitive protein-binding assay for 25-hydroxycholecalciferol. Clin Sci Mol Med. 1974 Feb;46(2):231–240. doi: 10.1042/cs0460231. [DOI] [PubMed] [Google Scholar]
- Goodman D. S. Vitamin A transport and retinol-binding protein metabolism. Vitam Horm. 1974;32:167–180. doi: 10.1016/s0083-6729(08)60011-4. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Birge S. J. Widespread, specific binding of 25-hydroxycholecalciferol in rat tissues. J Biol Chem. 1975 Jan 10;250(1):299–303. [PubMed] [Google Scholar]
- Haddad J. G., Chyu K. J. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1971 Dec;33(6):992–995. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Hillman L., Rojanasathit S. Human serum binding capacity and affinity for 25-hydroxyergocalciferol and 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1976 Jul;43(1):86–91. doi: 10.1210/jcem-43-1-86. [DOI] [PubMed] [Google Scholar]
- Haddad J. G., Jr, Walgate J. 25-Hydroxyvitamin D transport in human plasma. Isolation and partial characterization of calcifidiol-binding protein. J Biol Chem. 1976 Aug 25;251(16):4803–4809. [PubMed] [Google Scholar]
- Haddad J. G., Stamp T. C. Circulating 25-hydroxyvitamin D in man. Am J Med. 1974 Jul;57(1):57–62. doi: 10.1016/0002-9343(74)90768-2. [DOI] [PubMed] [Google Scholar]
- Hillman L. S., Haddad J. G. Human perinatal vitamin D metabolism. I. 25-Hydroxyvitamin D in maternal and cord blood. J Pediatr. 1974 May;84(5):742–749. doi: 10.1016/s0022-3476(74)80024-7. [DOI] [PubMed] [Google Scholar]
- Holick M. F., DeLuca H. F. A new chromatographic system for vitamin D3 and its metabolites: resoluation of a new vitamin D3 metabolite. J Lipid Res. 1971 Jul;12(4):460–465. [PubMed] [Google Scholar]
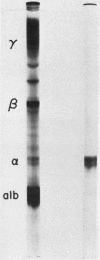
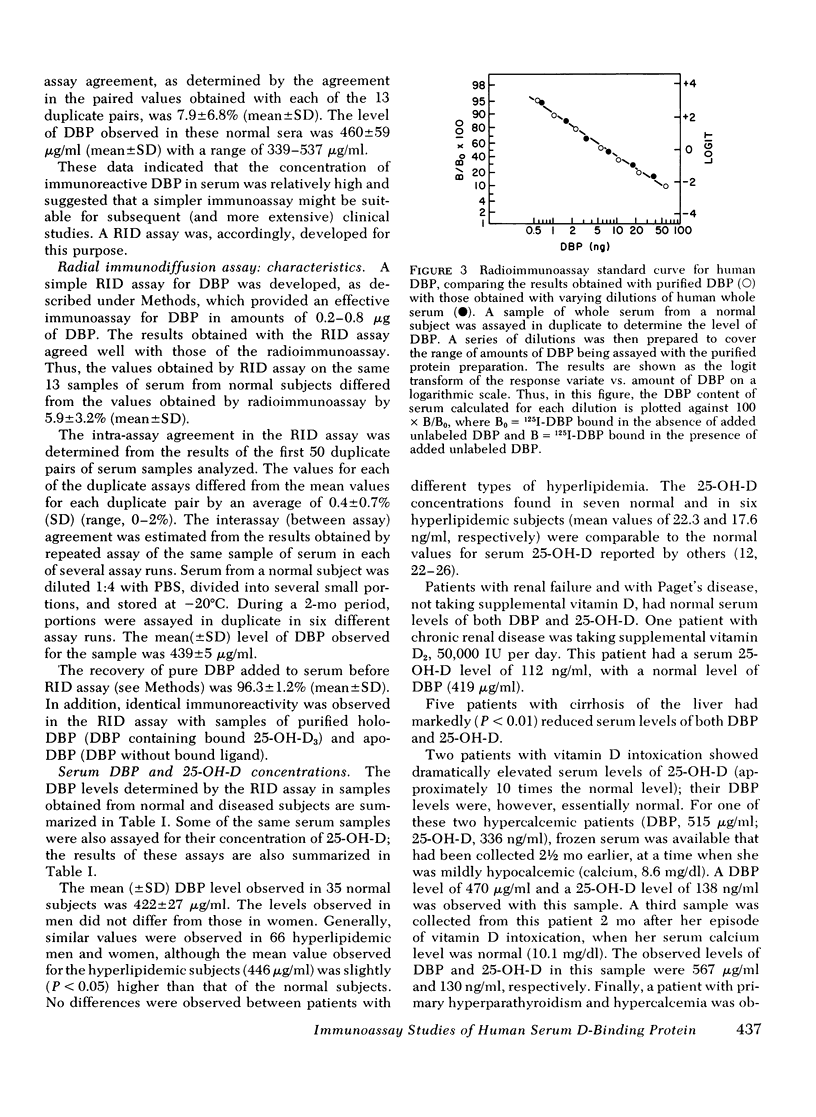
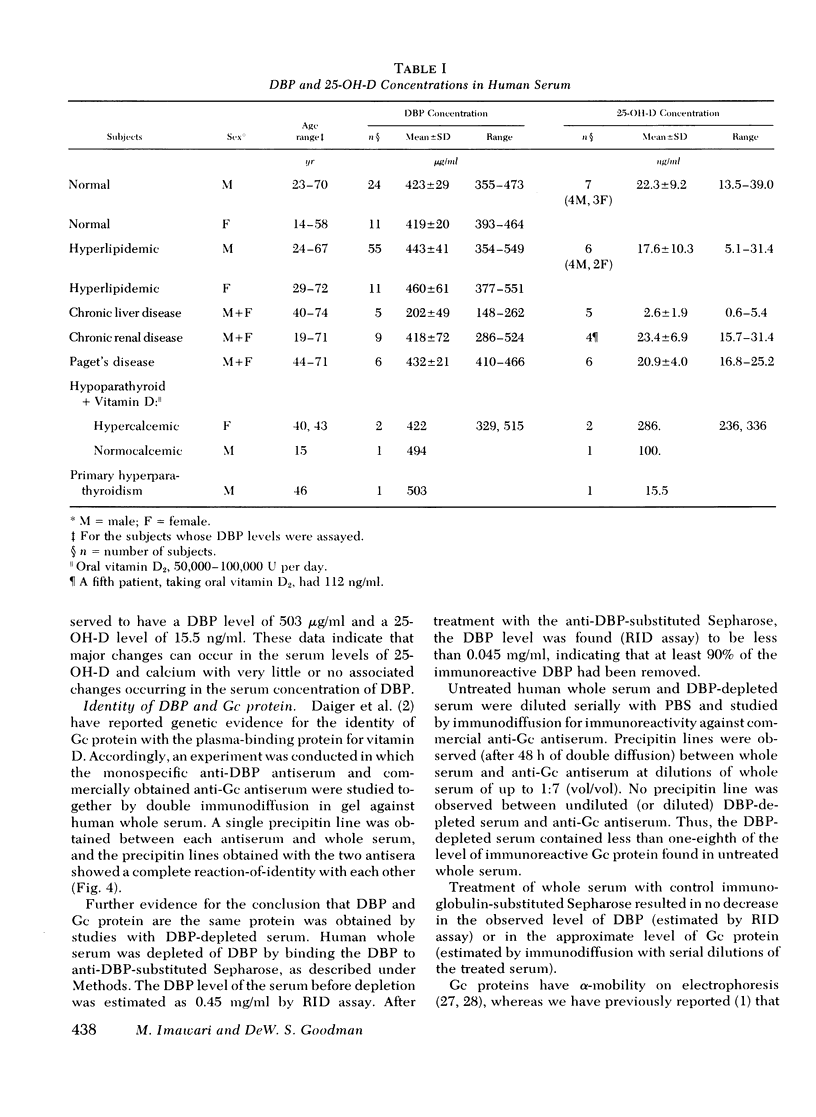
- Imawari M., Kida K., Goodman D. S. The transport of vitamin D and its 25-hydroxy metabolite in human plasma. Isolation and partial characterization of vitamin D and 25-hydroxyvitamin D binding protein. J Clin Invest. 1976 Aug;58(2):514–523. doi: 10.1172/JCI108495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITCHIN F. D., BEARN A. G. QUANTITATIVE DETERMINATION OF THE GROUP SPECIFIC PROTEIN IN NORMAL HUMAN SERUM. Proc Soc Exp Biol Med. 1965 Feb;118:304–307. doi: 10.3181/00379727-118-29827. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McLaughlin M., Raggatt P. R., Fairney A., Brown D. J., Lester E., Wills M. R. Seasonal variations in serum 25-hydroxycholecalciferol in healthy people. Lancet. 1974 Mar 30;1(7857):536–538. doi: 10.1016/s0140-6736(74)92717-2. [DOI] [PubMed] [Google Scholar]
- Miyachi Y., Vaitukaitis J. L., Nieschlag E., Lipsett M. B. Enzymatic radioiodination of gonadotropins. J Clin Endocrinol Metab. 1972 Jan;34(1):23–28. doi: 10.1210/jcem-34-1-23. [DOI] [PubMed] [Google Scholar]
- Muto Y., Goodman D. S. Vitamin A transport in rat plasma. Isolation and characterization or retinol-binding protein. J Biol Chem. 1972 Apr 25;247(8):2533–2541. [PubMed] [Google Scholar]
- Muto Y., Smith F. R., Goodman D. S. Comparative studies of retinol transport in plasma. J Lipid Res. 1973 Sep;14(5):525–532. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- PRUNIER J. H., BEARN A. G., CLEVE H. SITE OF FORMATION OF THE GROUP-SPECIFIC COMPONENT AND CERTAIN OTHER SERUM PROTEINS. Proc Soc Exp Biol Med. 1964 Apr;115:1005–1007. doi: 10.3181/00379727-115-29101. [DOI] [PubMed] [Google Scholar]
- Ponchon G., Kennan A. L., DeLuca H. F. "Activation" of vitamin D by the liver. J Clin Invest. 1969 Nov;48(11):2032–2037. doi: 10.1172/JCI106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Dingle J. T., Mallia A. K., Goodman D. S. The localization of retinol-binding protein in rat liver by immunofluorescence microscopy. J Cell Sci. 1975 Nov;19(2):379–394. doi: 10.1242/jcs.19.2.379. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Bridson W., Rayford P. L. Rapid calculation of radioimmunoassay results. J Lab Clin Med. 1969 Nov;74(5):770–781. [PubMed] [Google Scholar]
- Simons K., Bearn A. G. The use of preparative polyacrylamide-column electrophoresis in isolation of electrophoretically distinguishable components of the serum group-specific protein. Biochim Biophys Acta. 1967 Apr 11;133(3):499–505. doi: 10.1016/0005-2795(67)90554-5. [DOI] [PubMed] [Google Scholar]
- Smith F. R., Dell R. B., Noble R. P., Goodman D. S. Parameters of the three-pool model of the turnover of plasma cholesterol in normal and hyperlipidemic humans. J Clin Invest. 1976 Jan;57(1):137–148. doi: 10.1172/JCI108253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F. R., Goodman D. S. The effects of diseases of the liver, thyroid, and kidneys on the transport of vitamin A in human plasma. J Clin Invest. 1971 Nov;50(11):2426–2436. doi: 10.1172/JCI106741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. E., Goodman D. S. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J Clin Invest. 1971 Oct;50(10):2159–2167. doi: 10.1172/JCI106710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston P. D. A specific antiserum to lysosomal cathepsin D. Immunology. 1969 Sep;17(3):421–428. [PMC free article] [PubMed] [Google Scholar]