Summary
It is a well established norm that biomedical research involving human participants must conform to acceptable scientific principles and international codes of research ethics. The University of Ibadan/University College Hospital Health Research Ethics Committee (UI/UCH HREC) is the body that plays an oversight role and performs the function of a third party independent review of research protocols submitted by staff and students of the two institutions. A 6-year (2002–2007) retrospective audit of the protocols submitted to the HREC was performed to determine the profile of the lead investigator, sources of funding for the research and the duration for review using a 25 item questionnaire. A total of 752 protocols were submitted, 618 protocols (82%) were approved while 38 protocols were not approved. The principal investigators were mainly postgraduate students (67.1%) while academic staff constituted 21.3%. The average time from submission to approval was approximately 21 weeks (95% CI: 20 – 23 weeks). The period from submission to approval is significantly affected by the number of revision required and the funding agent (p < 0.05); it took a shorter time to review internationally funded research.
Keywords: Research ethics committee, research protocol, an appraisal, review process
Introduction
Ethical review is an important part of modern biomedical research. While the requirement for ensuring that research in human is conducted ethically has gained ground, reports continue to emerge of unethical conduct in different parts of the world. Modern norms of ethical review of research include several elements that are considered necessary to make research conduct ethical. These include review by independent “ethical committee”.
Until the early 19th century, medical interventions or experimentation on human beings was uncontrolled and unregulated. Thomas Percival (1740–1804), a physician from Manchester in England, elaborated what arguably is the first modern code of medical ethics in 1803 when he prescribed good methods and competent investigators, but was silent on ethics and informed consent.1 The first person to mention informed consent was William Beaumont (1785–1853), a surgeon in the US army. William Beaumont who became known as the “Father of Gastric Physiology” because of his research on human digestion underlined the need for a methodological approach as opposed to a random approach in experimentation.2
Research ethics committees (REC) originated from recommendations contained in the 1979 Belmont Report issued by the United States of America (USA) agency known as The National Commission for the Protection of Human Subjects of Biomedical and Behavioural Research.3 Ethical committee review of research was also included in the 1975 revision of the Declaration of Helsinki at the 29th World Medical Assembly in Tokyo.4 Human subject abuse scandals in the USA became widely known through a 1966 article of Henry K. Beecher, a professor of Anaesthesia at the Harvard Medical School.5 In the article, Beecher listed and described 22 clinical studies which had violated basic ethical principles of research on human beings. In the 1975 revision of the Declaration of Helsinki, committee review was mentioned in Principle 1.2: 4
The design and performance of each experimental procedure involving human subjects should be clearly formulated in an experimental protocol which should be transmitted to a specially appointed independent committee for consideration, comment and guidance.
However, despite the existence of international codes of ethics and institutional REC, unethical scientific enquiries have continued in many countries including Nigeria; for instance, the Trovan study conducted by Pfizer in Kano, Nigeria in 1996. The purpose of this study was to determine the effectiveness of Trovan in treating epidemic meningococcal meningitis. Pfizer, the manufacturer of Trovan, claimed it was not possible to gain consent from all parent because of the life-threatening epidemic, and the low literacy in the community. Following investigation on the activities of Pfizer during this epidemic by the Nigerian government, the panel concluded that Pfizer never obtained authorization from the Nigerian government to give the unproven drug to nearly 100 children and infants. Pfizer’s experiment was pronounced an illegal trial of an unregistered drug by a US law court where hearing on the case had continued.6
The REC is expected to play an oversight role and provides a third party independent review of research protocols to ensure safety of research participants and adherence to international codes of ethics including the Nuremberg Code, the Declaration of Helsinki, the Belmont Report and the Council for International Organizations for Medical Sciences (CIOMS) guidelines.7
Drawing on the basic philosophies underlying major ethical codes and declarations relevant to research with human subjects, Emanuel and colleague8 proposed seven requirements which make clinical research ethical. These requirement include: enhancement of health or knowledge, the research must be methodologically rigorous, the human subjects must be fairly selected based on the scientific objectives and not vulnerability or privilege or the potential for and distribution of risks and benefits, there must be favourable risk-benefit ratio within the context of standard clinical practice and the research protocol, an independent review by unaffiliated individuals to approve, amend, or terminate the research and an informed voluntary consent by individual participants. In addition, the human subjects should have: their privacy protected, the opportunity to withdraw, and their well-being monitored.
Materials and Methods
The University of Ibadan/University College Hospital, Ibadan, Nigeria Health Research Ethics Committee (UI/UCH HREC) is one of the research ethics committees in Nigeria established in 2002.7 The Committee reviews and approves as appropriate all research protocols involving human participants conducted by staff and students of the two institutions. A published review of the activities of the UI/UCH HREC over a 3 year period following its establishment revealed that 500 applications were received and the average period between protocol submission and approval decreased from 7.87 months in 2002, to 3.69 months in 2005.9 Additional information in this study include: the type and features of protocol submitted for review, the variation in approval time and reasons for amendments and disapproval.
In this study we conducted a retrospective review of all proposals submitted to the UI/UCH HREC during a 6-year period (2002–2007). Data about each protocol received between 2002 and 2007 was extracted using a 25-item questionnaire developed by the authors. The questionnaire has three sections. Section one extracted information on the month and year of submission of the protocol and the dates on which different determinations were made about the protocol. Section two obtained information on the academic status of the principal investigator of the research; whether the research was sponsored and who the sponsor was; location of conduct of the study, nature of the study; whether clinical, public health or laboratory based. Other questions in this section inquired about study design, participants’ characteristics, statistical analysis section, expected study duration and study benefits and incentives to participants. Other questions inquired about the number of revisions required before approval, reasons for revision or modifications where these were requested, the time interval between submission and approval; number of amendments after approval and approval status of the protocol at the time of this review.
The data was analyzed using STATA® (Statacorp 4905, Lakeway College Station, Texas 77845, USA), version 10. Categorical data were presented as proportions and using frequency distribution. Student t-test was used to compare the mean time from submission of protocols to approval, for protocols granted exempt approval and protocols requiring review.
Results
Number of protocol reviewed
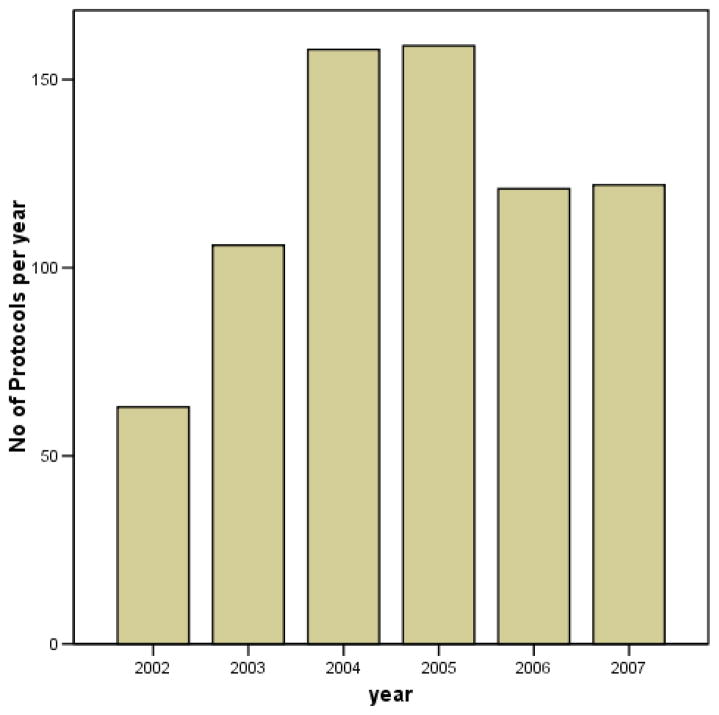
A total of 752 protocols were submitted to the UI/UCH Health Research Ethics Committee (HREC) within the study period. Of these, 728 (97%) records were retrieved for this audit. Of the 728 protocols audited, 56 protocols (0.08%) were still under consideration by the Committee at the time of the review while decision has been taken on 656/728 (90%) protocols. Most, 618 (94%) were approved; while 38 (6%) were not approved. The number of protocols submitted each month is shown in figure 1.
Figure 1.
Bar Chart showing number of protocols submitted per year
Academic Status of Principal Investigator and Source of Funding
With regards to the 656 protocols that were decided by the Committee during the period under review, the principal investigators were mainly postgraduate students (440/656, 67.1%) whereas, undergraduate students constituted 11.6% (76/656) and academic staff constitutes 21.3% (140/656). However, studies by undergraduate and postgraduate students were conducted under the supervision of academic staff. Only 89 (13.6%) of approved protocols were sponsored by funding agencies including international organizations (9.8%, 65/656), institutional research grants (1.5%,10/656), pharmaceutical industries (1.7%, 11/656) and other public institutions in Nigeria (0.46%, 3/656) as shown in Table 1.
Table 1.
Sources of funding
| Cadre | Source of funding | Total | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Undergraduate | 76 | - | - | - | - | - | 76 |
| Graduate | 436 | 2 | - | - | 1 | 1 | 440 |
| Lecturer | 15 | 2 | 2 | 4 | 1 | 24 | |
| Senior Lecturer | 30 | 6 | 8 | 1 | 24 | 2 | 71 |
| Professor & Ass. Prof | 6 | - | 1 | 2 | 36 | - | 45 |
| Total | 563 | 10 | 11 | 3 | 65 | 4 | 656 |
Key: 1=self funded, 2= institutional research grant, 3=pharmaceutical company, 4=other public institution, 5= international, 6= not stated
Location of the conduct and nature of study
The research site was a teaching hospital in 359 (54.7%) studies; 32 studies (4.9%) were conducted simultaneously in a teaching hospital and other health facilities, while the rest were within a community, educational institution, or laboratory based as shown in Table 2. Clinical studies constitute the bulk of the protocols submitted accounting for 44.9% (277/656), followed by studies in public health 19.8% (122/656), laboratory based studies 19.3% (119/656) and 6.9% (43/656) were on drug evaluations.
Table 2.
Location of study
| Site | N | % |
|---|---|---|
| Tertiary Teaching Hospital (TTH) | 359 | 54.7 |
| TTH and others | 32 | 4.9 |
| Rural community | 25 | 3.8 |
| Urban community | 117 | 17.8 |
| Urban and rural community | 9 | 1.4 |
| Primary or Secondary health facility | 15 | 2.2 |
| University and other tertiary institution | 72 | 11 |
| Primary/Secondary schools | 21 | 3.2 |
| Specialized laboratory | 3 | 0.5 |
| Others | 3 | 0.5 |
| Total | 656 | 100 |
The item “others” includes the prison, a beverage company and a conference venue.
Study design and Participants’ Characteristics
Studies were mainly descriptive (observational) in 500/656 instances (76.2%), experimental, non-randomised in 12.8% and experimental randomised (clinical trial) in 11%. Half (36/72) of the experimental randomised studies were conducted by senior academic staff (senior lecturer and above) and 40% (29/72) were conducted by postgraduate students. Out of the 618 protocols approved by the Committee, 553 (89.5%) were studies involving human participants recruited by investigators, 56 (9%) were on hospital records (case-note, treatment sheet and other vital records), 2 (0.3%) on cadavers, 2 (0.3%) on laboratory animals and 5 (0.8%) on hospital buildings or facilities as shown in Table 3.
Table 3.
Research Participants
| Research Subject | N | % |
|---|---|---|
| Healthy Adults | 197 | 31.9 |
| Healthy children | 22 | 3.6 |
| Adult patient | 270 | 43.7 |
| Paediatric patient | 51 | 8.2 |
| Adult & paediatric patient | 3 | 0.5 |
| Adult patient & Healthy volunteers | 10 | 1.6 |
| Hospital records | 56 | 9.1 |
| Laboratory animals | 2 | 0.3 |
| Human corpses | 2 | 0.3 |
| Hospital facilities | 5 | 0.8 |
| Total | 618 | 100 |
Reviews and Reasons for Revision
Only 5.3% (33/618) of the protocols were approved without request for modifications by the Committee while 464 (75.1%) required minor modifications after first review. Another 19.1% (118/618) required a second review while 0.5% (3/618) required a third review by the HREC. Donor-funded research usually required less number of revisions as 71.9% (64/89) required one revision while 28.1% (25/89) required at least two revisions, though this was not statistically significant.
The main reasons for requesting revision of protocols were inadequate information to the research participants as contained in the informed consent (283 protocols) inadequate description of the research methodology and method of statistical analysis (271 protocols), poor scientific justification (177 protocols), inadequate sample size calculation or justification (153 protocols), lack of clear inclusion criteria (72 protocols), inadequate treatment information (62 protocols), study objectives not clearly stated (34 protocols), and legal requirements for registration of drugs with the appropriate agency and others as shown in Table 4.
Table 4.
Reasons for revision before approval (n = 566)
| Reasons | No of Protocols | % |
|---|---|---|
| Patient information on consent form | 283 | 25.4 |
| Methodology and statistics | 271 | 24.3 |
| Scientific justification | 177 | 15.9 |
| Sample size justification | 153 | 13.7 |
| Inclusion criteria | 72 | 6.5 |
| Treatment information | 62 | 5.6 |
| Study objectives | 34 | 3.1 |
| Legal requirements | 15 | 1.4 |
| Confidentiality | 33 | 3.0 |
| Typographical errors | 8 | 0.7 |
| Incentive | 4 | 0.4 |
| Total | 1112* | 100 |
More than one reason per protocol reviewed
Approval and duration of Review
Some 10 protocols (1.6%) were exempt, 42 (6.8%) received expedited review while the remaining 566 (91.6%) protocol required full committee reviews and modifications before approval as shown in Table 5. In general, the average time from submission to approval was approximately 21 weeks (95% CI: 20 – 23 weeks); Protocols approved without further revision took 6 weeks on average (n = 33, 95% CI: 4 – 8 weeks) while donor-funded protocols took an average of 16 weeks (n = 64, 95% CI: 12 – 20 weeks). The need for revision significantly affect the time taken from submission of research proposal to approval, p <0.001 and also the number of revision required p< 0.05.
Table 5.
Time from submission to approval
| No of revisions | N | Mean (weeks) | Standard deviation | 95% Confidence Interval | Range (weeks) |
|---|---|---|---|---|---|
| 0 | 33 | 6.2 | 6.1 | 4.1 – 8.4 | 1 – 26 |
| 1 | 464 | 21.2 | 18.6 | 19.5 – 23 | 1 – 156 |
| 2 | 118 | 25.9 | 18.3 | 23 – 29 | 4 – 108 |
| 3 | 03 | 28.6 | 2.3 | 23 – 34 | 26 – 30 |
Discussion
There are few literatures examining the functioning of REC with regards to the nature and characteristics of protocols submitted to them. In this study, the yearly protocol submission increased by 155% from initial 62 in 2002 by year 2004 and 2005, the period during which a series of training workshops on research ethics were conducted in the twin institutions.10 In comparison, an audit by Cookson on the workload of a local REC in Leicestershire over a 10 year period revealed a steady rise in the number of protocols from 66 per year to 302.11 Majority of the proposals reviewed by the UI/UCH HREC were submitted by graduate and undergraduate students, though studies were conducted under the supervision of academic staff. The main reason for this finding is that resident doctors are required to obtain the local HREC committee approval to do a research project in their final examinations leading to the award of fellowship by the Postgraduate Medical Colleges. Another reason that could account for the large numbers of proposals from students is the need to publish research findings in reputable journals that would demand for the local HREC approval before publication of such.
We are surprised that academic staff members constitute less than a quarter of the lead investigators for the study protocols received during the period under review. A number of factors may account for this. First, it is possible that academic staff seek approval for only donor-funded research because of the requirements from donors. Secondly, staff may be shopping for approval from other existing REC available in the metropolis. Thirdly, many staff members are reluctant to subject their study proposal for ethical review due to lack of understanding of the role of HREC in the research project.10 Providing information and education on the role of HREC as an independent reviewer of a research proposal to prospective investigators and scientists is thus required. Certificate in research ethics may be made a pre-requisite for employment into residency training or admission for postgraduate degree in the two institutions.
There were few donor-funded studies in this review with over 80% of the studies being self-funded. Internationally funded projects constitute less than 10% of total donor-funded projects indicating the need for increase funding of research especially those being conducted by students. Funding of students’ studies could be achieved through creation of a research grant office by various teaching hospitals to support resident doctors or engagement of postgraduate students in donor-funded studies being conducted by senior academic staff. Seeking international collaboration for research in Nigeria is a daunting challenge due to poor health infrastructure, lack of standard of care for many disease conditions and the prevailing poor economy and poverty in all ramifications.
Most of the proposed studies reviewed were mainly hospital based. These findings affirmed the fact that it is probably easier to conduct studies in hospital setting because of ready access to hospital patients. The preponderance of hospital patients as research participants (52.4%) raises valid ethical and moral issues. For example, physicians may find their obligation as health care provider to individual patient come into conflict with their role as investigators. Some authors have also raised concerns about the difficulties of obtaining truly informed and truly voluntary consent from patients in health facilities.12 To avoid exploitation of hospital patient and undue influence, strict adherence to the 3 principles (Respect for autonomy, beneficence and Non-maleficence) guiding ethical conduct of research must be ensured.
A major finding in this study is that over 75% required minor modifications before approval compared to 57% reported by Decullier and colleague.13 Also, in a 12-month review by Cookson 51.6% were approved without amendment while 33.6% required minor amendments.11 The need for revision and number of times a protocol is reviewed significantly affect the time from submission of research proposal to approval. As noted by Ahmed and Nicholson14 in their study involving multi-centre studies, delay in obtaining approval from local HREC is related to the frequency with which ethics committees meet, and also their workload. The UI/UCH HREC meets once a month to review the protocols submitted for review, unfortunately, the limitation of this study is that HREC workload was not determined. However, the time taken from submission to approval can be improved through training of researchers, trainees and other staff cadre in Good Clinical Practice, research ethics, study design and research methodology. This training should be made available locally and should be affordable to the undergraduate and graduate students that constitute the bulk of investigators patronizing the UI/UCH HREC.
As found by this study, it takes an average of 21 weeks for a protocol to pass through the full review process; this is contrary to the NHREC {section E (d) (5)} guideline which stipulates a maximum period of 3 months from the date of receipt of a valid application.15 Independent review appears to take longer time at the UI/UCH HREC when compared to other findings from other countries, Dal-Re and colleague16 reported that that it takes 64 days from submission to approval of protocol in Spain while it takes a mean of 27 days in France.12 Approval for donor- funded research took a shorter period (16 weeks) compared to self-funded research (21 weeks). The probable reasons for this observation include the fact that internationally funded protocols are better-written, are conducted by senior members of staff who respond more quickly to reviewers’ comment because of tight deadlines and fear of losing the grant.
In conclusion, findings in this study indicate that there is a need to improve on the review process in order to reduce delays. The protocol review process at the UI/UCH HREC could be improved through provision of research ethics training to prospective principal investigators and increasing the number of trained reviewers.
Acknowledgments
This work was supported by Grant Number D43 TW007091 from the United States’ National Institutes of Health (NIH), Fogarty International Center and the National Human Genome Research Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the awarding office of the NIH/Fogarty International Center.
Contributor Information
Olayinka R. Eyelade, Department of Anaesthesia, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Ademola J. Ajuwon, Department of Health Education and Health promotion, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Clement A. Adebamowo, West African Bioethics Training Program, Department of Surgery, College of Medicine, University of Ibadan, Ibadan, Nigeria.
References
- 1.Editorial. Thomas Percival (1740 – 1804) Codifier of Medical Ethics. JAMA. 1965;194(12):1319–1320. [PubMed] [Google Scholar]
- 2.Lock S. Research ethics – a brief historical review to 1965. J Intern Med. 1995;238:513– 520. doi: 10.1111/j.1365-2796.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 3.National Commission for the protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report. Washington DC: US Government Printing Office; 1979. [Google Scholar]
- 4.Carlson RV, Boyd KM, Webb DJ. The revision of the Declaration of Helsinki: past, present, and future. Br J Clin Pharmacol. 2004;57:695–713. doi: 10.1111/j.1365-2125.2004.02103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beecher HK. Ethics and Clinical Research. NEJM. 1966;274(24):1354–1360. doi: 10.1056/NEJM196606162742405. [DOI] [PubMed] [Google Scholar]
- 6.Annas JG. Globalized clinical trials and informed consent. NEJM. 2009;360(20):2050–2053. doi: 10.1056/NEJMp0901474. [DOI] [PubMed] [Google Scholar]
- 7.Falusi AG. The UI/UCH Ethical Review Committee: Operations and Challenges. National Bioethics Training Workshop Proceedings; 2004; Funded by Ralph & Marion Falk Clinical Cancer Trust, U.S.A. [Google Scholar]
- 8.Emanuel EJ, Wedler D, Grady C. What Makes Clinical Research Ethical? JAMA. 2000;238(20):2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 9.Falusi AG, Olopade OI, Olopade CO. Establishment of a standing Ethics/Institutional review board in a Nigerian university: A blueprint for developing countries. JERHRE. 2007;2:21–30. doi: 10.1525/jer.2007.2.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Ajuwon AJ, Kass N. Outcome of a research ethics training workshop among clinicians and scientists in a Nigerian university. BMC Medical Ethics. 2008–9;1 doi: 10.1186/1472-6939-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cookson JB. Auditing a research ethics committee. J R Coll Physicians Lon. 1992;26(2):181–183. [PMC free article] [PubMed] [Google Scholar]
- 12.Abdool Karim Q, Abdool Karim SS. Informed consent for HIV testing in a South African hospital: is it truly informed and truly voluntary? Am J Public Health. 1998;88(4):637–652. doi: 10.2105/ajph.88.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deculier E, Lheriter V, Chapuis FR. The Activity of French Research Ethics Committees and Characteristics of Biomedical Research Protocols involving humans: A Retrospective Cohort Study. BMC Biomedical Ethics. 2005;6:9. doi: 10.1186/1472-6939-6-9. retrieved Mar. 18, 2008 from http://www.biomedcentral.com\content\6\1\9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed AH, Nicholson KG. Delays and diversity in the practice of local research ethics committees. J Med Ethics. 1996;22:263–266. doi: 10.1136/jme.22.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Code of Health Research Ethics. National Health Research Ethics Committee of Nigeria (NHREC) Federal Ministry of Health, Department of Health Planning and Research; 2007. Retrieved Nov. 19, 2007 from http://www.nhrec.net. [Google Scholar]
- 16.Dal-Re R, Espada J, Ortega R. Performance of research ethics committees in Spain. A prospective study of 100 applications for clinical trial protocols on medicines. J Med Ethics. 1999;25:268– 273. doi: 10.1136/jme.25.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]