Abstract
Treatment of chronic pain is associated with high variability in the response to pharmacological interventions. A mathematical pharmacodynamic model was developed to quantify the magnitude and onset/offset times of effect of a single capsaicin 8% patch application in the treatment of painful diabetic peripheral neuropathy in 91 patients. In addition, a mixture model was applied to objectively match patterns in pain-associated behavior. The model identified four distinct subgroups that responded differently to treatment: 3.3% of patients (subgroup 1) showed worsening of pain; 31% (subgroup 2) showed no change; 32% (subgroup 3) showed a quick reduction in pain that reached a nadir in week 3, followed by a slow return towards baseline (16% ± 6% pain reduction in week 12); 34% (subgroup 4) showed a quick reduction in pain that persisted (70% ± 5% reduction in week 12). The estimate of the response-onset rate constant, obtained for subgroups 1, 3, and 4, was 0.76 ± 0.12 week−1 (median ± SE), indicating that every 0.91 weeks the pain score reduces or increases by 50% relative to the score of the previous week (= t½). The response-offset rate constant could be determined for subgroup 3 only and was 0.09 ± 0.04 week−1 (t½ 7.8 weeks). The analysis allowed separation of a heterogeneous neuropathic pain population into four homogenous subgroups with distinct behaviors in response to treatment with capsaicin. It is argued that this model-based approach may have added value in analyzing longitudinal chronic pain data and allows optimization of treatment algorithms for patients suffering from chronic pain conditions.
Keywords: diabetic neuropathic pain, capsaicin 8%, modeling, mixture model
Introduction
The development of painful diabetic peripheral neuropathy (DPN) is a common long-term complication in diabetic patients. It is relatively more common in older patients and patients with suboptimal glycemic control.1 Although the exact pathophysiologic mechanism is unknown, several contributing factors have been proposed, such as microvascular insufficiency, oxidative stress, nitrosative stress, defective neurotrophism, and autoimmune-mediated nerve destruction.2,3 Patients with DPN exhibit a variety of pain symptoms and sensory qualities. Approximately 20%–24% of patients experience onset of insidious pain (or dysesthesias) and present with varying degrees of numbness, tingling, burning pain, loss of sensations, paresthesias and loss of balance or coordination. Of the 60% of diabetics who develop neuropathy, about 30%–40% have no symptoms.4
Attempts to treat DPN can be divided into those directed at modification of the underlying disease process and those directed toward symptom suppression.5 No consensus on the optimal management of neuropathic pain exists and consequently the treatment of pain is largely empirical and diverse, relying primarily on antidepressants, anticonvulsants and narcotic analgesics.6 This study focuses on the local application of a high concentration (8%) capsaicin patch, NGX-4010 (Qutenza™) for treatment of painful DPN. Capsaicin is the pungent ingredient in chili peppers and it is believed that exaggerated activity of capsaicin-sensitive nerve fibers is involved in the pain of peripheral neuropathies like DPN and postherpetic neuralgia.7 Capsaicin is a highly selective activating ligand for transient receptor potential vanilloid 1 (TRPV1), which is a ligand-gated nonselective cation channel highly expressed in small diameter primary afferent neurons (C- and Aδ-fibers), especially those nerve fibers that specialize in the detection of painful or noxious sensations,8–10 Activation of this receptor by capsaicin results in a burning sensation, hyperalgesia, allodynia, and erythema (due to release of vasoactive neuropeptides from small-diameter sensory axons).9 After prolonged exposure to capsaicin, the small diameter sensory axons become less sensitive to a variety of stimuli, including capsaicin itself or thermal stimuli, resulting in a reduced pain response.8 These later stage effects of capsaicin are frequently referred to as “defunctionalization” and serve as the rationale for the development of capsaicin formulations for the treatment of various neuropathic pain syndromes.7 Studies have shown that such alterations from prolonged low-concentration capsaicin exposure are reversible, at which point normal function (the detection of noxious sensations) returns.11,12
In this study, patients received a single treatment with the high concentration capsaicin patch (NGX-4010). While a (limited) descriptive analysis of the data has been published previously,20 an independent re-analysis was performed, using nonlinear mixed effect modeling in NONMEM considering the whole time course of effect (0–12 weeks).14 The aims of the study were (i) to get an indication of the variability in responses and attempt to identify subgroups in response to capsaicin treatment using a pharmacodynamic mixture model; (ii) to describe the magnitude of effect and time courses of onset and offset of effect of 8% capsaicin patch in the observed subgroups.
Methods
The study was registered in the Clinical Trial register (www.clinicaltrials.gov) under number NCT00082316.
Patient population and study design
After approval of the protocol by the local ethics committee patients with moderate to severe pain (numerical pain rating score [NPRS] of 3 or greater on a scale from 0 [no pain] to 10 [worst possible pain]), secondary to DPN were enrolled in the study. DPN was defined as neuropathic pain (related to type I or II diabetes mellitus) in both feet for at least 3 months prior to the study.
Inclusion criteria were: men and women aged at least 18 years, absence of pain from other causes (eg, from fibromyalgia, arthritis, mononeuritis multiplex, hereditary neuropathy), intact skin around the treatment area, absence of significant medical problems of the heart, kidneys, liver, or lungs. Chronic pain medication was allowed with the exception of any topical medication in the affected areas of the body, including nonsteroidal anti-inflammatory drugs, menthol, methyl salicylate, local anesthetics, steroids, or capsaicin. After the screening visit, no pain medication changes were allowed. Exclusion criteria included a history of substance abuse, pregnancy or lactation, the presence of cancer, opioid medication use, unless orally or transdermally administered and not exceeding a total daily dose of morphine 60 mg/day, chronic alcohol abuse, uncorrected vitamin B12 deficiency, or treatment with any drugs that may have contributed to the neuropathy during the 90 days prior to the study, hypersensitivity to capsaicin (ie, chili peppers or over-the-counter capsaicin products), local anesthetics or adhesives.
This multicenter study had a randomized, open-label design and was aimed at the evaluation of the tolerability and efficacy of the application of 1–4 high-concentration capsaicin (640 μg/cm2) patches, preceded by the topical application of lidocaine 4%. Prior to treatment with NGX-4010, the lidocaine cream was applied over and extending 1–2 cm beyond the perimeter of the marked painful area(s) for 60 minutes. After removal of the topical anesthetic, up to four 20 × 14 cm capsaicin patches were applied for 60–90 minutes. The patch(es) were then removed and a cleansing gel was used to remove excess capsaicin from the skin, after which the treated area was gently washed with soap followed by water. The patients were monitored for at least 2 hours following treatment before being discharged. For discomfort, the patients were permitted to use analgesic medications during (oxycodone oral solution 1 mg/mL) and after treatment (hydrocodone/acetaminophen tablets, 5/500 mg, two tablets every 8 hours, for a maximum of 5 days).
The primary study parameter was the average NPRS for the past 24 hours obtained at 24 hour intervals at 0900 PM. Average weekly NPRSs were used in the analysis, without imputation for missing scores. Pain scores were obtained for 12 weeks following the application of the capsaicin patch.
Pharmacodynamic data analysis
Model fitting was performed using a nonlinear mixed effects modeling approach using nonlinear mixed effect modeling software (NONMEM version VII level 1; ICON Development Solutions, Ellicott City, MD).14 The first-order conditional estimation (FOCE) with interaction algorithm was used for model development. The performance of the analysis was evaluated by various selection criteria, including visual inspection of the goodness-of-fit plot, changes in the objective function value and parameter estimates and their respective standard errors. Using the likelihood ratio test, the significance level was set at α = 0.01, which corresponds to a reduction of 6.6 units in objective function value (χ2 distribution) to discriminate between two nested structural models after inclusion of one additional parameter. Model diagnostic checks of the final model were conducted using R (R-project, version 2.12.0).
Pharmacodynamic model
The effect of capsaicin 8% on NPRS was characterized using a Bateman function with the following structure: Effect
| (1) |
The Bateman function characterizes the time course of NPRS pain score from week 0 (baseline) to week 12 in terms of two first-order rate constants describing the onset (konset) and offset (koffset) of the effect following the application of capsaicin 8%. The change in NPRS from week 0 to week 12 was computed as follows:
| (2) |
where NPRS0 is the NPRS pain score at baseline and α is the magnitude of effect.
Random effects were included in the pharmacodynamic model provided that the parameters are either normally or log-normally distributed. For example, the pharmacodynamic parameter α was modeled assuming a normal distribution allowing for the estimation of negative values of the parameter α (increase in NPRS pain score):
| (3) |
where αj is the estimate of α for the jth individual and θα represents the population estimate for the pharmacodynamic parameter α. ηj describes the inter-individual variability on α, which is assumed to be a normally distributed random effect variable with mean zero and variance ω2. Similarly, the inter-individual random effect variables for NPRS0 and konset were assumed to be normally distributed. The interindividual variability random effect variable for koffset was assumed to be log-normally distributed.
Residual variability, which is a measure of the unexplained variability (including error associated with reporting of chronic pain outcome), was described using an additive error model:
| (4) |
where the residual variability, ɛij, at time point i for individual j, is a normally distributed random effect variable with mean zero and variance σ2.
Mixture model
Aside from the advantage of the use of the population approach in simultaneously analyzing individual data, the nonlinear mixed effects modeling approach also enables the definition of a mixture model, in which the existence of subgroup of patients that may respond differently to capsaicin 8% patch treatment can be explored. For this analysis, it is assumed that the structural model is the same for subgroups, but that patients may differ in their onset and offset of response and also may respond differently to capsaicin 8% patch in terms of magnitude of response. To this end, the pharmacodynamic parameters, konset, koffset and α are assumed to be distributed multimodally and therefore the mixture feature in NONMEM is used to differentiate between subgroups of patients on the basis of the distribution of the pharmacodynamic parameter estimates. Initially, based on visual inspection of the individual data, the mixture model was defined to separate four subgroups of patients, including patients that show (1) worsening of response, (2) no response, (3) maximum response with trend to return to baseline after 12 weeks and (4) maximum response which is maintained during 12 weeks.
Results
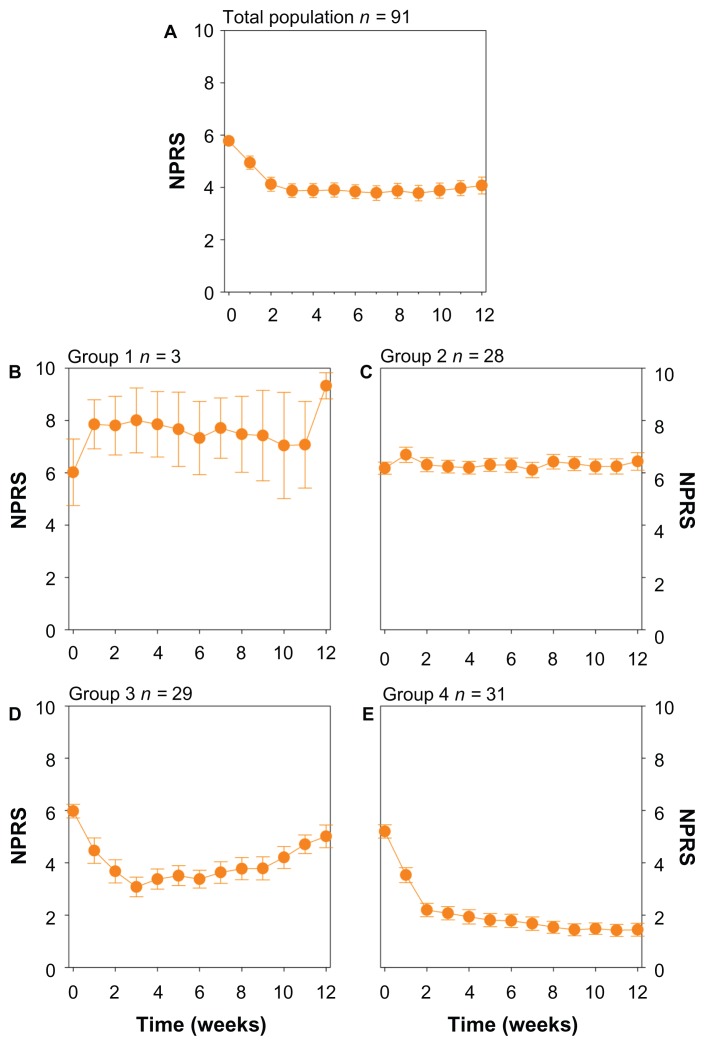
The data from 91 patients were included in the analysis. Data of patients with early termination from the study were included in the analysis (n = 13). Early termination was most often related to unsatisfactory therapeutic response and occurred most often in week 6 of the study. An overview of the demographic patient data (of patients included in the analysis) is provided in Table 1. The mean time course profiles of the numerical pain rating score for the total population are shown in Figure 1A. The mean percentage of pain reduction from baseline at week 12 for the total population was 30.5% (95% confidence interval [CI]: 21.1%–39.9%). The proportion of patients that show at least 30% reduction in NPRS from baseline at week 12 was 46.8%; the 50% responder rate at week 12 was 33.8%.
Table 1.
Demographics of patients involved in the analysis
| Characteristic | % of population |
|---|---|
| Number of patients | 91 |
| Sex distribution (n) | |
| Men | 52 (57.1%) |
| Women | 39 (42.9%) |
| Age ± SD (years) | 58.7 ± 11.21 |
| Age distribution (n) | |
| <65 years | 63 (69.2%) |
| ≥65 years | 28 (30.8%) |
| Weight ± SD (kg) | 97.7 ± 22.86 |
| H eight ± SD (cm) | 172.5 ± 9.81 |
| Race distribution (n) | |
| White | 69 (75.8%) |
| African American | 10 (11.0%) |
| Hispanic | 9 (9.9%) |
| Other | 3 (3.3%) |
Abbreviation: SD, standard deviation.
Figure 1.
(A) Mean response of the total population (n = 91). (B) Mean response of patients belonging to group 1 (patients with a deterioration of their pain) as determined from the mixture model analysis. (C) Mean response of patients belonging to group 2 (patients with no response to treatment). (D) Mean response of patients belonging to group 3 (patients with an initial drop in NPRS followed by a slow decline towards baseline NPRS). (E) Mean response of patients belonging to group 4 (patients with a reduction in NRPS which is maintained throughout the study period). Values are mean ± SEM.
Abbreviations: NPRS, numerical pain rating score; SEM, standard error of the mean.
Description of the four subgroups
The mixture model analysis clearly showed that the dataset can be separated into subgroups (Tables 2–4). These subgroups have different shapes of the time versus response profile, indicating that patients differ in their response to capsaicin 8% treatment. The mean time course profiles of NPRS pain score for the different subgroups are shown in Figure 1B–E. The standard errors shown serve to illustrate that the average NPRS score in each subgroup (except for subgroup 1) is estimated with a high degree of precision as indicated by the relative low standard errors.
Table 2.
Pharmacodynamic parameter estimates
| Subgroup | Typical estimate ± SE | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Fixed effect parameters (θ) | ||||
| NPRS0 | 6.0 ± 0.1 | 6.0 ± 0.2 | 6.0 ± 0.2 | 6.0 ± 0.2 |
| konset (week−1) | 0.76 ± 0.12 | 0 ± NE | 0.76 ± 0.12 | 0.76 ± 0.12 |
| koffset (week−1) | 0 ± NE | 0 ± NE | 0.09 ± 0.04 | 0 ± NE |
| α | −0.2 ± 0.08 | 0 ± NE | 0.79 ± 0.06 | 0.79 ± 0.06 |
| Interindividual random effect parameters | ||||
| NPRS0 (%CV) | 2.04 ± 0.323 (24%) | |||
| konset (%CV) | 0.20 ± 0.08 (58%) | |||
| koffset (%CV) | 1.30 ± 0.75 (114%) | |||
| α (%CV) | 0.20 ± 0.08 (subgroup 1: 232%; 2–4: 57%) | |||
| Residual random effect parameter | ||||
| Additive residual error | 0.51 ± 0.082 | |||
Abbreviations: NPRS0, numerical pain rating score at baseline; konset, the responseonset rate constant; koffset, the response-offset rate constant; SE, standard error; α, magnitude of effect.
Patients in subgroup 1 (n = 3) displayed a worsening of response (increase in NPRS score) following capsaicin 8% treatment that was maintained during the 12 weeks of the study (Figure 1B). The mean increase in NPRS from baseline at week 12 was 28.0%. Note, however, that this subgroup contains just three subjects.
Subgroup 2 contains 28 patients that showed no or minimal response to capsaicin 8% treatment (Figure 1C). On average, a slight increase in NPRS pain score from baseline of 5.62% was observed (Table 2), while none of the patients in that subgroup showed at least 30% reduction in NPRS from baseline at week 12.
Subgroup 3 and 4 do show a clear analgesic response to capsaicin 8% treatment. On average, the 29 patients in subgroup 3 had a quick reduction in NRPS with a nadir of 42.6% reduction in NPRS occurring between weeks 3 and 4, followed by a slow increase NPRS towards baseline (Figure 1D). At the end of the study the mean NPRS reduction was 15.6%, while the proportion of patients that showed at least 30% reduction in NPRS pain score was 34.8%; the 50% responder rate was 13.0%.
Patients in subgroup 4 show a sharp reduction in NPRS that was maintained during the 12 weeks of the study ( Figure 1E). The mean percentage reduction in NPRS from baseline is 69.7% at week 12% and 90.3% of the patients showed at least 30% reduction in NPRS pain score from baseline at week 12, while 74.2% of the patients showed at least 50% reduction in NPRS pain score from baseline.
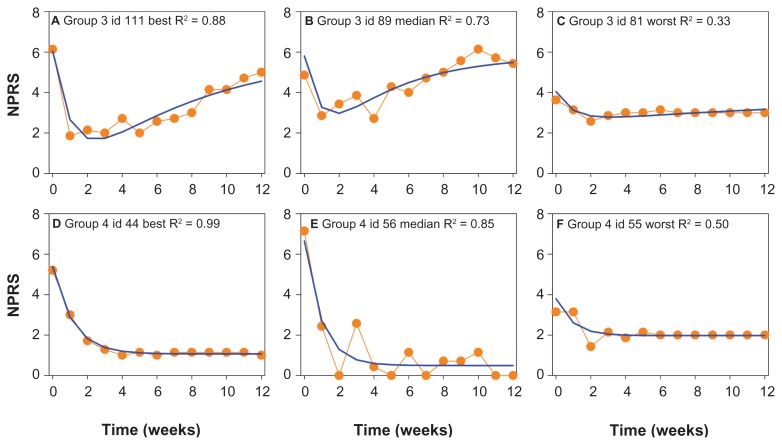
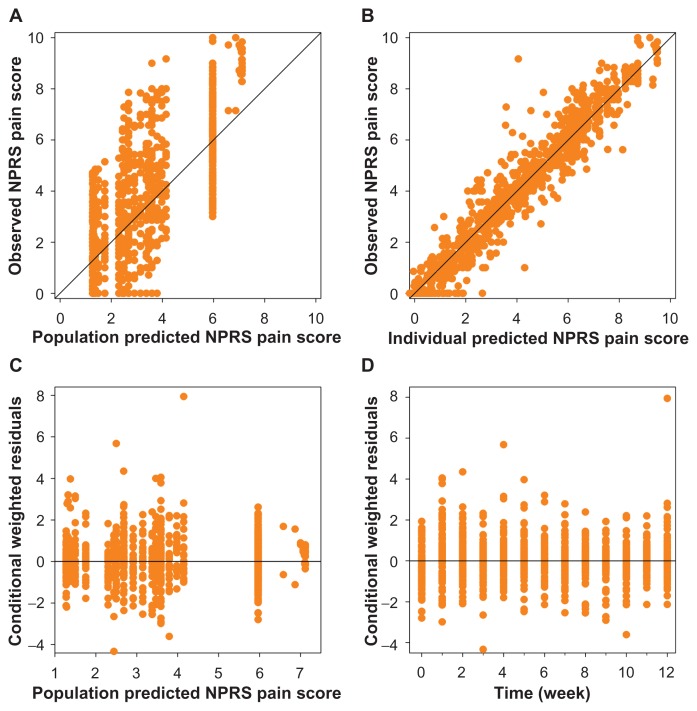
To get an indication of the adequacy of the model, best, median and worst data fits of NPRS obtained in groups 3 and 4 are given in Figure 2. The goodness-of-fit plots given in Figure 3 do not show any systemic deviation of the model predicted versus observed NPRS.
Figure 2.
Examples of the data fits of Groups 3 and 4. Best, median and worst fits of NPRS responses belonging to Groups 3 (A, B, and C) and 4 (D, E, and F) are given.
Abbreviation: NPRS, numerical pain rating score.
Figure 3.
Goodness-of-fit plots of the final pharmacodynamic model. Observed versus population predicted (A) and individual predicted NPRS pain score (B). The black lines are the lines of identity. In the lower panel the conditional weighted residuals versus population predicted NPRS score (C) and time (D) are plotted.
Abbreviation: NPRS, numerical pain rating score.
Pharmacodynamic parameter values
Speed of onset
These distinct profiles are translated into quantitative estimates of pharmacodynamic parameters, which may differ across the different subgroups. For subgroups 1, 3, and 4, the rate of onset was estimated at 0.76 week−1 (Table 2), corresponding to a half-life (t1/2,onset) of 0.91 weeks, indicating that every 0.91 weeks the NPRS is reduced or increased (in case of subgroup 1) by 50% relative to the NPRS obtained in the previous week. Differences in onset of effect between the subgroups has been formally tested for during the analysis, but did not result in a further improvement of the model fit at the level of α = 0.01, indicating that the rate of onset of response is similar for the subgroups. For subgroup 2, the patients that showed no or minimal response to capsaicin 8% treatment, the rate constant for onset of effect was assumed to be 0 and was based on the reasonable assumption that this subgroup has no kinetics of action. In total 28 patients of 91 patients (30.8%) showed no effect to capsaicin 8% treatment.
Speed of offset
The offset of effect was quite different between the subgroups. Patients in subgroup 3 (n = 29, 31.9%) showed a response to capsaicin 8% patch treatment, which gradually returned toward baseline. The estimate of the rate constant of offset for subgroup 3 was 0.09 week−1 (Table 2), corresponding to a half-life (t1/2,offset) of 7.8 weeks (based on the 12-week treatment period). This indicates that every 7.8 weeks the NPRS is increased by 50% relative to the NPRS of the previous week. Subgroups 1, 2 and 4 did not have an offset of effect. For subgroup 4 (n = 31, 34.1% of patients) this is shown in Figure 1E by a well-preserved maintenance of the maximum response during the 12 study weeks, showing no offset of effect. For subgroup 1 (n = 3, 3.3% of patients), NPRS pain scores go up and remain at higher scores after treatment with capsaicin 8%.
Discussion
The development of effective and safe treatments for chronic neuropathic pain indications remains challenging. The heterogeneity (eg, ethnicity, age, sex) and complexity (eg, disease processes, underlying mechanisms) of the chronic pain population, including DPN patients, is well recognized and may partly explain the large variability in the response to pharmacological treatment.15,16 In the current study a mathematical pharmacodynamic model was developed, considering the time course of response, to quantify the magnitude and onset/offset times of the effect of capsaicin 8% patch in the treatment of DPN. In addition, a mixture model was applied to objectively match patterns in pain-associated behavior (NPRS). The main findings of the study are: (i) on average, a single application of capsaicin 8% patch produced a variable analgesic response with an average 30.5% pain reduction (95% CI: 21.1%–39.9%) from baseline NPRS at week 12 in DPN patients; and (ii) using the pharmacodynamic mixture model, four distinct subgroups could be identified that respond differently to capsaicin 8% treatment. Subjects allocated to group 1 (3.3% of patients) showed worsening of pain; group 2 (31%) showed no change in NPRS from baseline pain; group 3 (32%) had a quick drop in NPRS with a nadir between weeks 3 and 4, followed by a slow return towards baseline; group 4 (34%) had a quick reduction in NPRS that was maintained throughout the 12 weeks of the study (Figure 1; Table 2). With the exclusion of group 1, each of the groups displayed a significant reduction in response variability separating a heterogeneous population into homogenous subgroups in terms of response to treatment. This further underlines the relevance of collecting time course data during and after the treatment period and provides confidence that the developed pharmacodynamic model adequately described the time course of response in individual patients.
The division into the four subgroups was based on characterization of the time course of the response in terms of onset/offset times and magnitude of response. The onset of response had a half-life of about 1 week that was similar for groups 3 and 4 and also group 1. In the latter group the response was algesic rather than analgesic. This indicates that the onset of efficacy is relatively fast and within 3–4 weeks maximum reduction in NPRS was achieved (3–4 × t½onset). Group 1 includes just three patients and cannot be considered a robust response group compared to groups 2, 3, and 4. It is not possible to understand the algesic behavior in this small group. Possible causes include the presence of unpredictable and varying pain in these patients with spontaneous worsening of symptoms irrespective of treatment, or the presence of mood-related disorders with a poor or erratic response to any medication.
In group 3, NPRS was reduced by 42.6% from baseline at week 4 after treatment (30% and 50% responder rates were 60.7% and 42.9%, respectively, Table 3). In group 4, reduction in NPRS from baseline was 61.7% at week 4 after treatment (30% and 50% responder rate were 83.9% and 64.5%, respectively, Table 4). The response to capsaicin 8% patch treatment was further maintained or even increased from week 5 to at least week 12 in this subgroup, indicating long-lasting pain relief from a single patch application. Only in group 3 a response–offset rate constant could be estimated with a half-life of 7.8 weeks. This indicates that in group 3 the NPRS increased slowly (≈50% increase every 7.8 weeks from week 4 on), suggesting that patients in group 3 would need retreatment after 10 to 12 weeks (at that time the reduction in NPRS is less than 20%). Furthermore, a design in which retreatment is applied would enable assessment of stable treatment reactions.
Table 3.
Percentage reduction in numerical pain rating score from baseline at week 4 and 12
| Percentage of total population | Week 4 % (mean ± SE) |
Week 12 % (mean ± SE) |
|
|---|---|---|---|
| Total population | 100 | 33.7 ± 3.99 | 30.5 ± 4.84 |
| Subgroups | |||
| Worsening of response (group 1) | 3.3 | −34.7 ± 10.3 | −28.0 ± 1.84 |
| No response (group 2) | 30.8 | 0.01 ± 2.12 | −5.62 ± 3.7 |
| Maximum response with trend to return to baseline (group 3) | 31.9 | 42.5 ± 5.94 | 15.6 ± 6.30 |
| Maximum response which is maintained during 12 weeks (group 4) | 34.1 | 61.7 ± 5.24 | 69.7 ± 5.11 |
Abbreviation: SE, standard error.
We previously assessed the effect of a 1-week ketamine intravenous treatment on neuropathic pain in patients with complex regional pain syndrome type 1 (CRPS1).17,18 At the end of the 12-week follow-up, about 20% of the subjects had significant pain relief (ie, relief >30% of baseline pain score versus 47% in the current study, Table 4). While this suggests a rather poor efficacy of ketamine in the treatment of CRPS1 pain a subsequent subgroup analysis using a pharmacodynamic mixture model showed that 37% (11/30) of patients were unresponsive to ketamine treatment following the treatment week (cf, our groups 1 and 2), 56% (17/30) of patients had an initial analgesic response (>50% of baseline pain) that slowly returned towards baseline levels (cf, our current group 3) and just 7% (2/30) patients had persistent pain relief (cf, group 4).7 Evidently, patients that were unresponsive to ketamine are not suitable for retreatment. In contrast, patients in the ketamine group 3 were given the opportunity for retreatment with ketamine. Similarly, in our current study, patients in group 3 would be well served by retreatment.
The current and previous analyses indicate that subdividing patients into specific subgroups provides additional information of the effect of pharmacological treatment on pain responses over time by identifying homogenous subgroups with two classes of responders (ie, group 3 and 4 responders). This approach is superior to the dichotomous classification of patients into responders versus nonresponders made at a specific time-point following treatment (eg, at an arbitrary week following treatment). Also in our study, conclusions drawn form a dichotomous study outcome at week 12 would significantly differ from those obtained using the current approach and would have missed the earlier but transient robust analgesic effects and would have suggested the need for retreatment at week 12 in most patients. Retreatment of patients in subgroups 1 and 2 is not warranted, as discomfort will predominate without any therapeutic benefit. Retreatment in subgroup 4 is not needed since the analgesic response to the patch application was maintained during the observation period.
We restricted the mathematical modeling of the treatment responses to analgesia. We did not take side effects into account, as serious adverse events did not occur (side effects were transient capsaicin-application related, and included local erythema, pain, and itching).13 Still, for other types of systemic medication that show similar large variability in response efficacy (eg, NMDA receptor antagonists, GABAergic, antiepileptic and antidepressant medications) nontransient central side effects (sedation/nausea/dysphoria/hallucinations) may occur in patients that experience analgesia as well as those that do not.17,18 Especially for these medications with a narrow therapeutic index the characterization of subgroups is important as it allows a more accurate description of the efficacy–safety balance. Concomitant modeling of analgesia and side effects will allow the development of so-called utility functions in which the balance between safety and efficacy is quantified over time.19,20 For example patients that do not respond to medication (cf, our groups 1 and 2) are especially vulnerable to a potential imbalance between efficacy and safety as they lack any treatment advantage.
A limitation to our analysis is that in case of small response groups (subgroup 1 with just three patients), changes observed in effect could easily be due to random variation without any relationship to treatment. Furthermore, while our classification into four response groups is an important enrichment tool (eg, for assessment of treatment efficacy or an indicator for the need for treatment switch) it remains a post-hoc analysis. A useful addition to our analysis would be to incorporate a priori functional or neurosensory testing allowing response prediction. To the best of our knowledge, so far, no useful link has been made between any functional testing and drug response outcome. A first approach could be the use of quantitative sensory testing (QST) or brain-evoked potentials using electrical (SEP) or laser stimulation (LEP) of the skin prior to treatment. For example, specific QST, SEP, or LEP patterns detected may be linked to specific response groups. Our model-based approach can be seen as a complementary tool to functional tests like QST, combining mechanistic insights in chronic pain conditions with patient outcome measures. An additional approach could be to link response groups to patient covariates (including patient characteristics, genetic factors (eg, variations in the TRPV1 gene), disease severity/state. While this initially requires post-hoc testing, subsequent studies may be designed to address the prognostic value of these covariates in predicting the response to treatment. The developed pharmacodynamic model can be used as a basis for identification of covariates that have potential prognostic value for prediction of treatment outcome, our patient population, however, was too small to allow for such covariate analysis.
In conclusion, we showed that characterization of the time course of the analgesic response is essential to further understand the heterogeneity in treatment effect (and study population). In the current study in a relatively small population of neuropathic pain patients, about two-thirds of the patients showed significant reduction in pain following a single application of capsaicin 8% patch. In one subpopulation (34%) the 30% and 50% responder rate was around 90% and 70% at week 12. These results indicate that the single topical application of capsaicin 8% patch provides an effective and long-lasting treatment in DPN pain, although the efficacy should be viewed in light of the open-label study design. Finally, we and others,17,18 have shown that the model-based approach may have added value in analyzing longitudinal data from chronic pain trials and may provide insights into the nature of drug response and allows the optimization of treatment algorithms for patients suffering from chronic pain conditions. We applied the mixture model analysis to a small population of DPN patients treated with a single capsaicin 8% patch, but it may be a valuable tool for all therapies with similar large response variability in a variety of chronic pain syndromes, including ketamine, pregabalin, duloxetine, and gabapentin.17,21–23
Acknowledgments and disclosure
Ashraf Yassen, Paul Passier and Malcolm Stoker are employees of Astellas Pharma Global Development Europe, Leiderdorp, The Netherlands. Christian Martini, Erik Olofsen and Albert Dahan report no conflict of interest.
References
- 1.Spruce MC, Potter J, Coppini DV. The pathogenesis and management of painful diabetic neuropathy: a review. Diabetic Med. 2002;20:88–98. doi: 10.1046/j.1464-5491.2003.00852.x. [DOI] [PubMed] [Google Scholar]
- 2.Dyck PJ, Giannini C. Pathologic alterations in the diabetic neuropathies of humans: a review. J Neuropathol Exp Neurol. 1996;55:1181–1193. doi: 10.1097/00005072-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Vinik AI. Advances in diabetes for the millennium: new treatments for diabetic neuropathies. Med Gen Med. 2004;(Suppl 6):13. [PMC free article] [PubMed] [Google Scholar]
- 4.Baron R, Tölle TR, Gockel U, Brosz M, Freynhagen R. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. Pain. 2009;146:34–40. doi: 10.1016/j.pain.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus. JAMA. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 6.Koltzenburg M. Painful neuropathies. Curr Opinion Neurol. 1999;11:515–521. doi: 10.1097/00019052-199810000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Anand P, Bley K. Topical capsaicin for pain management: Therapeutical potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107:490–502. doi: 10.1093/bja/aer260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 9.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- 10.Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 11.Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135–145. doi: 10.1016/s0304-3959(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 12.Simone DA, Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilation in humans. Pain. 1991;47:285–294. doi: 10.1016/0304-3959(91)90217-L. [DOI] [PubMed] [Google Scholar]
- 13.Webster LR, Peppin JF, Murphy FT, Lu B, Tobias JK, Vanhove GF. Efficacy, safety, and tolerability of NGX-4010, capsaicin 8% patch, in an open-label study of patients with peripheral neuropathic pain. Diabetes Res Clin Prac. 2011;93:187–197. doi: 10.1016/j.diabres.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Beal BL, Sheiner LB, Boeckman AJ, Bauer RJ. NONMEM User’s Guide. Ellicott City, MD: Icon Development Solutions; 2009. [Google Scholar]
- 15.Christakis NA. Does this work for you? To say a drug “works” is only half the story. Brit Med J. 2008;337:1025. [Google Scholar]
- 16.Wolfe GI, Barohn RJ. Painful peripheral neuropathy. Curr Treat Options Neurol. 2002;4:177–188. doi: 10.1007/s11940-002-0034-0. [DOI] [PubMed] [Google Scholar]
- 17.Sigtermans M, van Hilten JJ, Bauer MCR, et al. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain. 2009;145:304–311. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Dahan A, Olofsen E, Sigtermans M, et al. Population pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain relief in chronic pain. Eur J Pain. 2001;15:258–267. doi: 10.1016/j.ejpain.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Yassen A, Olofsen E, Kan J, Dahan A, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the effectiveness and safety of buprenorphine and fentanyl in rats. Pharm Res. 2008;25:183–193. doi: 10.1007/s11095-007-9440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanderigo E, Sartori V, Sveticic G, et al. The well-being model: a new drug interaction model for positive and negative effects. Anesthesiology. 2006;104:742–755. doi: 10.1097/00000542-200604000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Lunn MPT, Hughes RAC, Wiffen PJ. Duloxetin for treating painful neuropathy or chronic pain. Cochrane Database Syst Rev. 2009;4:CD007115. doi: 10.1002/14651858.CD007115.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;3:CD007076. doi: 10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;3:CD007938. doi: 10.1002/14651858.CD007938.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]