Abstract
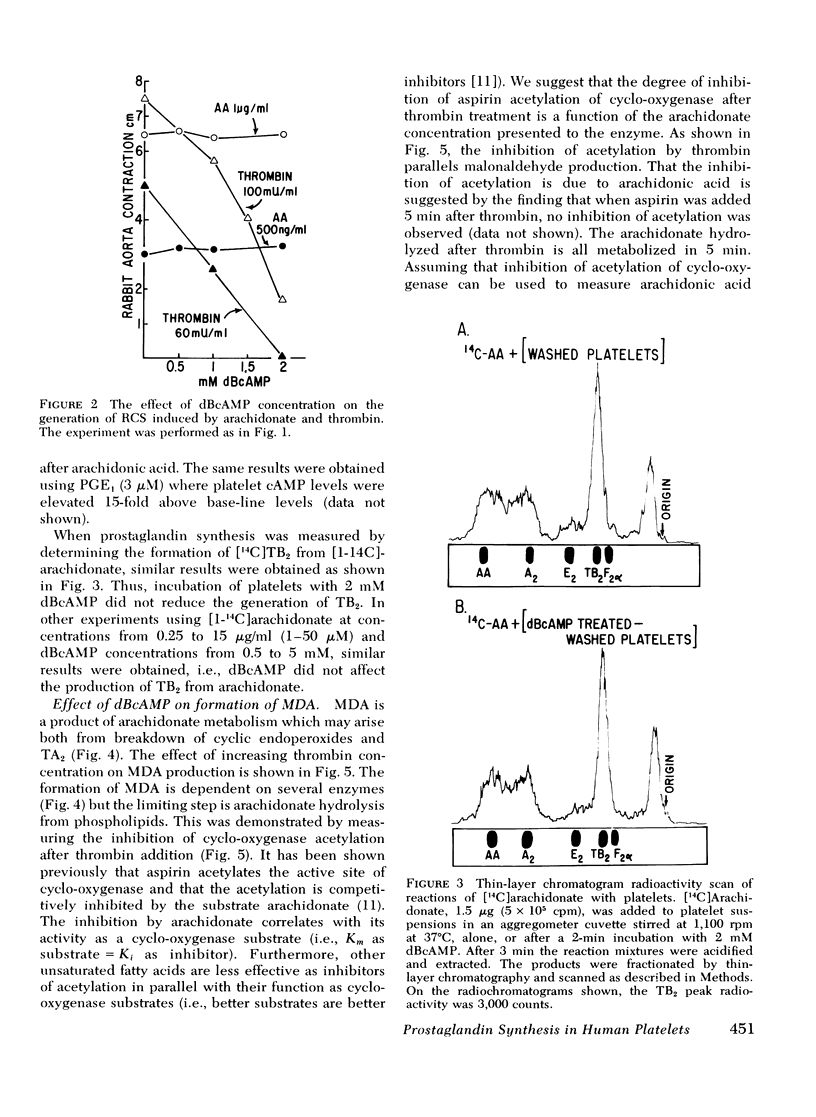
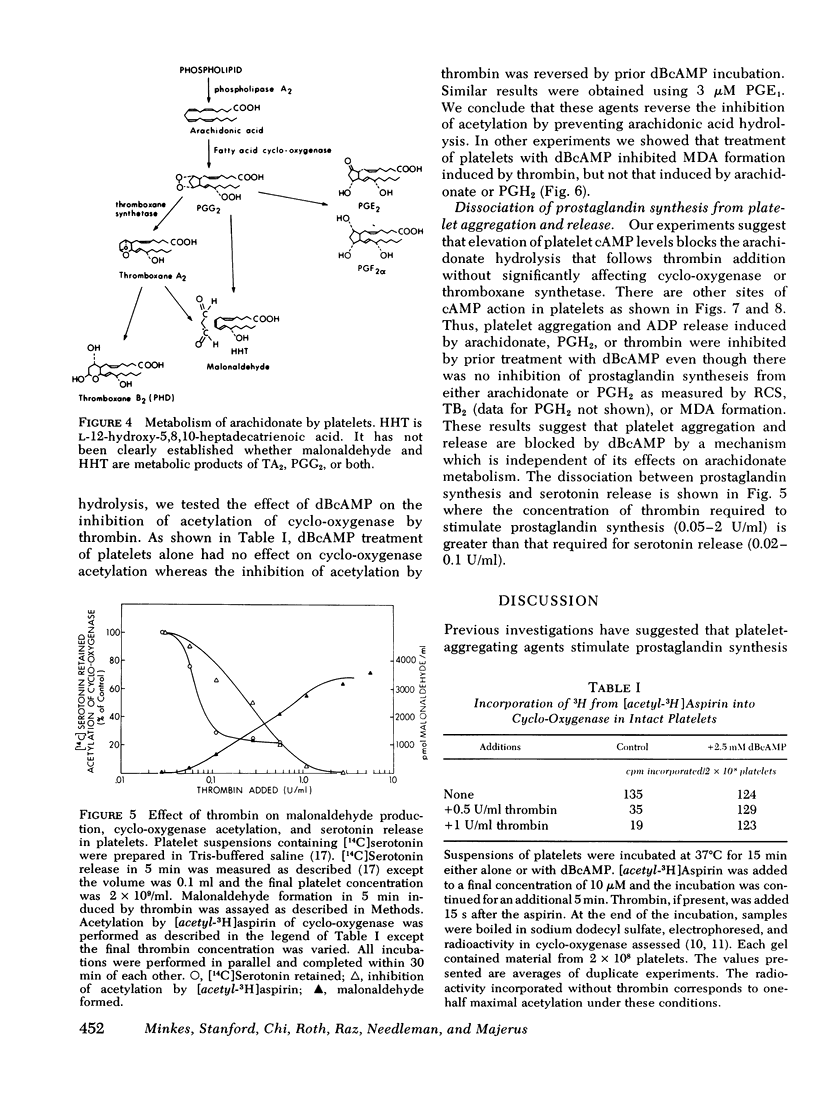
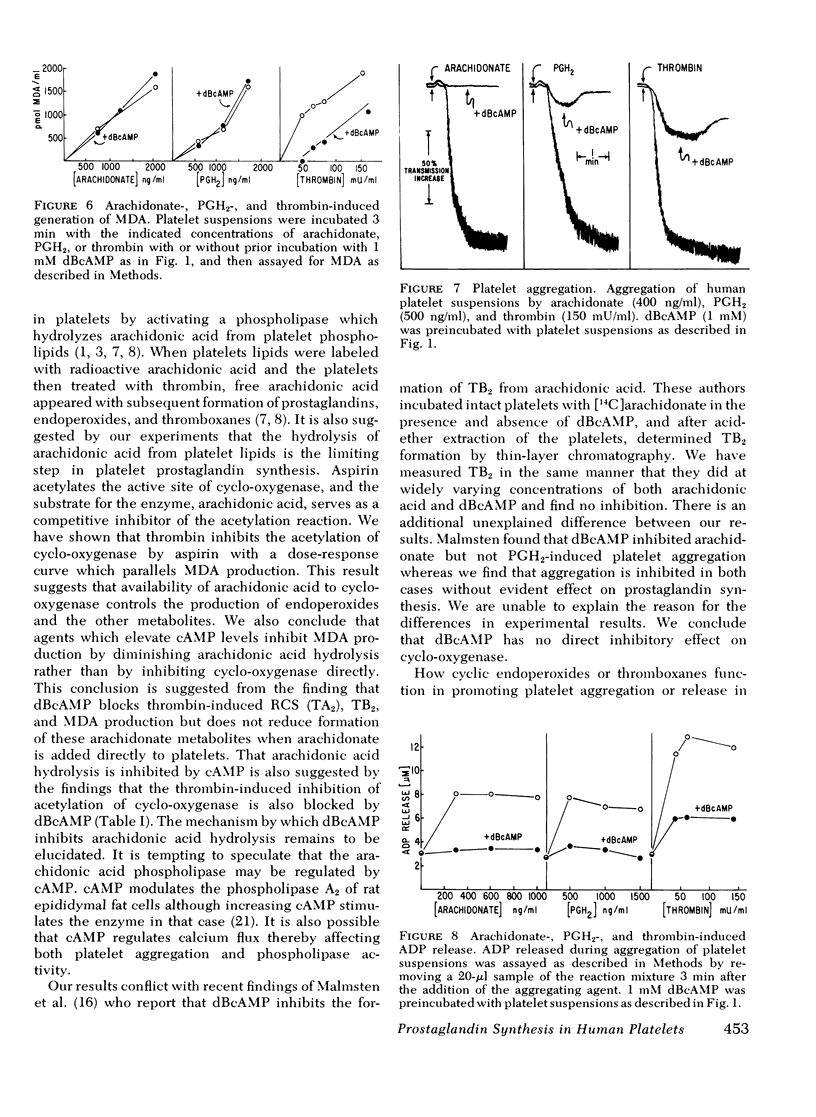
When thrombin is added to washed human platelets, one of its actions results in activation of a phospholipase that hydrolyzes arachidonic acid from phospholipids. The arachidonate is converted to the cyclic endoperoxides (prostaglandin G2 and prostaglandin H2) by fatty acid cyclo-oxygenase. These compounds are then converted to thromboxane A2, also called rabbit aorta-contracting substance, by thromboxane synthetase. These labile, pharmacologically active compounds then break down to inactive products including thromboxane B2 and malonaldehyde. Incubation of platelets with either dibutyryl cyclic adenosine 3',5'-monophosphate (dBcAMP) or prostaglandin E1 (PGE1) before thrombin addition blocks the subsequent formation of oxygenated products of arachidonic acid including thromboxane A2, thromboxane B2, and malonaldehyde. In contrast, when arachidonic acid is added directly to platelets, prior incubation with dBcAMP or PGE1 does not inhibit production of the prostaglandins or their metabolites. Thrombin treatment of platelets also blocks the acetylation of cyclo-oxygenase by aspirin since the hydrolyzed arachidonic acid competes with aspirin for the active site on cyclo-oxygenase. Prior treatment of platelets with dBcAMP or PGE1 reverses the thrombin inhibition of the acetylation of cyclo-oxygenase. We conclude that agents which elevate platelet cAMP levels inhibit the hydrolysis of arachidonic acid from platelet phospholipids. We also find that prostaglandin synthesis can be dissociated, in part, from platelet aggregation and release, and that cAMP has separate actions on these processes. Higher thrombin concentrations are required to stimulate prostaglandin synthesis (0.05-2 U/ml) than are required to induce [14C]serotonin release (0.02-0.1 U/ml). Furthermore, dBcAMP and PGE1 both inhibit platelet aggregation induced by either arachidonic acid or prostaglandin H2 without affecting the production of prostaglandin metabolites from these compounds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bills T. K., Smith J. B., Silver M. J. Metabolism of [14C]arachidonic acid by human platelets. Biochim Biophys Acta. 1976 Feb 23;424(2):303–314. doi: 10.1016/0005-2760(76)90198-3. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc Natl Acad Sci U S A. 1974 Feb;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam R. J. Interactions of the pharmacological receptors of blood platelets with adenylate cyclase. Ser Haematol. 1973;6(3):333–350. [PubMed] [Google Scholar]
- Haslam R. J. Roles of cyclic nucleotides in platelet function. Ciba Found Symp. 1975;35:121–151. doi: 10.1002/9780470720172.ch7. [DOI] [PubMed] [Google Scholar]
- Kaneshiro M. M., Mielke C. H., Jr, Kasper C. K., Rapaport S. I. Bleeding time after aspirin in disorders of intrinsic clotting. N Engl J Med. 1969 Nov 6;281(19):1039–1042. doi: 10.1056/NEJM196911062811904. [DOI] [PubMed] [Google Scholar]
- Majerus P. W. Editorial: Why aspirin? Circulation. 1976 Sep;54(3):357–359. doi: 10.1161/01.cir.54.3.357. [DOI] [PubMed] [Google Scholar]
- Malmsten C., Granström E., Samuelsson B. Cyclic AMP inhibits synthesis of prostaglandin endoperoxide (PGG2) in human platelets. Biochem Biophys Res Commun. 1976 Jan 26;68(2):569–576. doi: 10.1016/0006-291x(76)91183-9. [DOI] [PubMed] [Google Scholar]
- Miller O. V., Gorman R. R. Modulation of platelet cyclic nucleotide content by PGE1 and the prostaglandin endoperoxide PGG2. J Cyclic Nucleotide Res. 1976;2(2):79–87. [PubMed] [Google Scholar]
- Needleman P., Minkes M., Raz A. Thromboxanes: selective biosynthesis and distinct biological properties. Science. 1976 Jul 9;193(4248):163–165. doi: 10.1126/science.945611. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H., Hazelhof E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim Biophys Acta. 1973 Dec 20;326(3):448–461. doi: 10.1016/0005-2760(73)90145-8. [DOI] [PubMed] [Google Scholar]
- Roth G. J., Majerus P. W. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J Clin Invest. 1975 Sep;56(3):624–632. doi: 10.1172/JCI108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. J., Stanford N., Majerus P. W. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell F. A., Deykin D. The effect of thrombin on the uptake and transformation of arachidonic acid by human platelets. Am J Hematol. 1976;1(1):59–70. doi: 10.1002/ajh.2830010107. [DOI] [PubMed] [Google Scholar]
- Salzman E. W. Prostaglandins and platelet function. Adv Prostaglandin Thromboxane Res. 1976;2:767–780. [PubMed] [Google Scholar]
- Salzman E. W., Weisenberger H. Role of cyclic AMP in platelet function. Adv Cyclic Nucleotide Res. 1972;1:231–247. [PubMed] [Google Scholar]
- Shuman M. A., Tollefsen D. M., Majerus P. W. The binding of human and bovine thrombin to human platelets. Blood. 1976 Jan;47(1):43–54. [PubMed] [Google Scholar]
- Smith J. B., Ingerman C., Kocsis J. J., Silver M. J. Formation of an intermediate in prostaglandin biosynthesis and its association with the platelet release reaction. J Clin Invest. 1974 May;53(5):1468–1472. doi: 10.1172/JCI107695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D. M., Feagler J. R., Majerus P. W. The binding of thrombin to the surface of human platelets. J Biol Chem. 1974 Apr 25;249(8):2646–2651. [PubMed] [Google Scholar]
- Willis A. L., Vane F. M., Kuhn D. C., Scott C. G., Petrin M. An endoperoxide aggregator (Lass), formed in platelets in response to thrombotic stimuli: purification, identification and unique biological significance. Prostaglandins. 1974 Dec 25;8(6):453–507. doi: 10.1016/0090-6980(74)90062-8. [DOI] [PubMed] [Google Scholar]
- Zucker M. B., Peterson J. Effect of acetylsalicylic acid, other nonsteroidal anti-inflammatory agents, and dipyridamole on human blood platelets. J Lab Clin Med. 1970 Jul;76(1):66–75. [PubMed] [Google Scholar]
- Zucker M. B., Peterson J. Inhibition of adenosine diphosphate-induced secondary aggregation and other platelet functions by acetylsalicylic acid ingestion. Proc Soc Exp Biol Med. 1968 Feb;127(2):547–551. doi: 10.3181/00379727-127-32737. [DOI] [PubMed] [Google Scholar]
- de Cingolani G. E., van den Bosch H., van Deenen L. L. Phospholipase A and lysophospholipase activities in isolated fat cells: effect of cyclic 3',5'-AMP. Biochim Biophys Acta. 1972 Mar 23;260(3):387–392. doi: 10.1016/0005-2760(72)90053-7. [DOI] [PubMed] [Google Scholar]



