Abstract
Objective
Due to the rapid proliferation of human immunodeficiency virus (HIV) treatment options, there is a need for health care providers with knowledge of antiretroviral therapy intricacies. In a HIV multidisciplinary care team, the HIV pharmacist is well-equipped to provide this expertise. We conducted a systematic review to assess the impact of HIV pharmacists on HIV clinical outcomes.
Methods
We searched six electronic databases from January 1, 1980 to June 1, 2011 and included all quantitative studies that examined pharmacist’s roles in the clinical care of HIV-positive adults. Primary outcomes were antiretroviral adherence, viral load, and CD4+ cell count and secondary outcomes included health care utilization parameters, antiretroviral modifications, and other descriptive variables.
Results
Thirty-two publications were included. Despite methodological limitation, the involvement of HIV pharmacists was associated with statistically significant adherence improvements and positive impact on viral suppression in the majority of studies.
Conclusion
This systematic review provides evidence of the beneficial impact of HIV pharmacists on HIV treatment outcomes and offers suggestions for future research.
Keywords: pharmacist, HIV/AIDS, clinical, adherence, impact
Introduction
Since the first reported cases of AIDS in 19811 and the emergence of the global human immunodeficiency virus (HIV) pandemic, the field of antiretroviral (ARV) therapy has undergone extraordinary changes and continues to witness dramatic progress. The availability of over two dozen distinct ARVs, providing more tolerable and safer agents, and the ability to tailor ARV regimens to individual patients, demonstrates the substantial advancement in the field and the heightened understanding and expertise that is required to minimize drug interactions, contraindications, and adverse effects. The increased incidence of comorbidities in the aging HIV-positive population demands close monitoring and a keen awareness of the interplay between various therapies, the transmission of drug resistant viruses requires knowledge of ARV regimen selection, and the need for life-long therapy necessitates high ARV adherence and long-term follow-up. Therefore, the HIV clinical pharmacist has emerged as an indispensable member of the HIV multidisciplinary care team.
Publications as early as 1991 have described the involvement of pharmacists in clinics and hospital teams caring for HIV-positive individuals.2–4 These and other studies5,6 demonstrate the importance of the pharmacist’s medication expertise and involvement in the multidisciplinary care team. Most recently, Horberg et al7 examined the components of the HIV multidisciplinary care team that are associated with the greatest increases in ARV adherence. The involvement of clinical pharmacists represented the first branch of the regression tree (signifying the component of the care team with the greatest impact on adherence) and the presence of clinical pharmacists resulted in statistically significant improvements in adherence in conjunction with any multidisciplinary care team member.
Given the extensive history and indications that clinical pharmacists may be particularly valuable in the medical care of HIV-positive individuals, we conducted a systematic review to assess the contributions of HIV pharmacists on HIV clinical outcomes, including ARV adherence and virologic and immunologic parameters. The purpose of this review was to systematically evaluate the research conducted to date and identify gaps in our knowledge regarding the impact of HIV clinical pharmacists in the clinical care of those living with HIV/AIDS.
Methods
Objective
The primary objective of this systematic review was to evaluate the impact of clinical pharmacists on HIV clinical outcomes. Primary outcomes included ARV adherence, HIV viral load suppression, and CD4+ cell count. Secondary outcomes consisted of health care utilization parameters, antiretroviral modifications, and other descriptive variables.
Data sources
We searched PubMed, EMBASE®, Cochrane Library, Web of Science®, BIOSIS Previews, and PsycINFO® from 1980 (or the respective date of inception of each database) until June 1, 2011. Additionally, we conducted a manual search by screening the references of pertinent articles and identifying any additional relevant publications that were not previously included. Due to incomplete data presentation in conference abstracts, we did not include conference proceedings and abstracts in this review.
Search strategy
We conducted our search strategy in the style of Cochrane Highly Sensitive Search Strategy to identify all relevant published studies.8 We included randomized and nonrandomized controlled trials, before-after comparisons, historically controlled trials, cohort studies, cross-sectional studies, case-control studies, and descriptive studies, as well as appropriate medical subject headings (MeSH) terms, and a wide range of relevant search terms in all databases. The detailed search strategy used for PubMed can be found in Table 1. This strategy was modified as appropriate for use in other databases.
Table 1.
Example of search strategy used in PubMed
| Search # | PubMed search terms |
|---|---|
| #7 | Search #5 AND #6 |
| #6 | Search randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized controlled trials[mh] OR random allocation[mh] OR double-blind method[mh] OR single-blind method[mh] OR clinical trial[pt] OR clinical trials[mh] OR “clinical trial”[tw] OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) OR Placebos[mh] OR placebo*[tw] OR random*[tw] OR nonrandomi*[tw] OR before after study[tw] OR time series[tw] OR “case control”[tw] OR prospective*[tw] OR retrospective*[tw] OR cohort[tw] OR cross-section*[tw] OR research design[mh:noexp] OR comparative study[pt] OR evaluation studies[pt] OR follow-up studies[mh] OR prospective studies[mh] OR controlled[tw] OR control[tw] OR volunteer*[tw] OR longitud*[tw] OR descripti*[tiab] OR study[tiab] OR evaluat*[tiab] OR “odds ratio”[tw] OR “hazard ratio”[tw] OR “relative risk”[tw] OR “risk ratio”[tw] OR AOR[tiab] OR RRR[tiab] OR NNT[tiab] OR design*[tiab] |
| #5 | Search #1 AND #2 AND #3 AND #4 |
| #4 | Search HAART[tiab] OR ART[tiab] OR ARV[tiab] OR ARVs[tiab] OR anti-retroviral*[tiab] OR antiretroviral*[tiab] OR “antiviral”[tiab] OR antiviral[tiab] OR “anti-HIV” OR antiHIV OR “Antiretroviral Therapy, Highly Active”[mh] OR “Anti-Retroviral Agents”[mh] OR CD4[tw] OR immune[tw] OR immunolo*[tw] OR immunology[sh] OR lymphocyte[tw] OR CD4-Positive T-Lymphocytes[mh] OR CD4 Lymphocyte Count[mh] OR CD4 count* OR “viral load”[tw] OR virol*[tw] OR viral[tw] OR virology[sh] OR outcome OR outcomes OR prognosis OR “outcome and process assessment(health care)”[mh] OR th[sh:noexp] OR dt[sh] OR effective*[tw] |
| #3 | Search adhere*[tiab] OR complian*[tiab] OR adhere*[tw] OR complian*[tw] OR Patient Compliance[mh] OR Medication Adherence[mh] OR Counseling[mh] OR counsel*[tw] OR education[tw] OR monitor*[tw] OR interven*[tw] OR self administration[mh] |
| #2 | Search pharmacist*[tiab] OR PharmD[tiab] OR “Pharm D”[tiab] OR pharmacy[tiab] OR pharmacies[tiab] OR pharmacists[mh] OR pharmacy[mh] OR community pharmacy services[mh] OR medication therapy management[mh] OR pharmacy service, hospital[mh] OR pharmaceutical services[mh:noexp] |
| #1 | Search HIV Infections[mh] OR HIV[mh] OR HIV[tiab] OR HIV-1[tiab] OR HIV-2[tiab] OR HIV-1[tiab] OR HIV-2[tiab] OR HIV infect*[tiab] OR human immunodeficiency virus[tiab] OR human immune deficiency virus[tiab] OR human immuno-deficiency virus[tiab] OR ((human immun*) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immune deficiency syndrome[tiab] OR acquired immuno-deficiency syndrome[tiab] OR ((acquired immun*) AND (deficiency syndrome[tiab])) OR HIV/AIDS[tiab] OR “HIV AIDS”[tiab] OR “Sexually Transmitted Diseases, Viral”[mh] |
Inclusion and exclusion criteria
We included all studies that examined the role of a pharmacist in the clinical care of HIV-infected adults. Studies were divided into two broad categories based on the researchers’ prespecified intentions in examining the impact of pharmacists. The first category encompassed “intervention studies”; these studies included HIV pharmacist activities that were part of a study protocol and were only implemented for the purpose of research upon receipt of informed consent. The second category included studies of “clinical care activities”; defined as studies which examined pharmacist actions that were taken as part of routine patient care and which examined specific outcomes (eg, the impact of an existing pharmacist adherence clinic on adherence). These clinical care activities were not conducted for the purpose of research and would have occurred regardless of the study. The reason for this classification was to assess the rigor of the research and the evolution of publications regarding HIV clinical pharmacists over time. Studies that did not include details of the pharmacist’s involvement, but specifically mentioned any pharmacist participation were included. Multifactorial interventions or clinical care activities were included as long as at least one factor clearly indicated pharmacist contributions.
We also classified the pharmacist role as being central or peripheral to the study objectives. The pharmacist role was considered “central” in studies that were specifically designed to examine the influence of pharmacists on the care of HIV-positive individuals. Studies where the role of the pharmacist was “peripheral” were those in which the pharmacist was involved in carrying out the study objectives, but the research was not designed to examine the sole impact of the pharmacist.
We did not include studies that exclusively assessed pharmacist’s ability to provide HIV prevention services or studies that only assessed pharmacy operations (such as medication stock, home delivery, medication packaging, etc). Studies were included without regard to the location where they were conducted, but were limited to English language publications. Research that was purely qualitative was excluded.
Review methods and data abstraction
Using EndNote software package (X5.0.1; Thomson Reuters, New York, NY) relevant studies were located in the above-mentioned data sources and duplicates and irrelevant articles were extracted by one author (PS). Two authors (PS, JC) independently read the remaining citations and identified eligible studies based on prespecified inclusion/exclusion criteria. All uncertainties and disagreements were arbitrated by a third author (BD). Using a data abstraction form, three authors (PS, JC, BD) summarized pertinent information from included articles and over 30% of all abstracted data was re-examined by another author to ensure data accuracy. We utilized the Cochrane guide for study assessment checklist to assign the study design to each included study.9
Outcome variables
The primary outcome of this review focused on the impact of the pharmacist on ARV adherence, HIV viral load, and CD4+ cell count. Secondary outcomes included change in the number of physician or emergency room visits, change in pill burden (ie, frequency of daily dosing or quantity of pills per day), cost effectiveness or any cost containment data, discontinuation or initiation of opportunistic infection prophylaxis or treatment, percentage of clinical care activities accepted by the attending physician or team, change in patients’ or providers’ HIV knowledge, impact on ARV drug resistance, and reports of the number of clinical care activities conducted by the pharmacist (eg, identification of dose errors, initiation/discontinuation/consolidation of ARVs, adverse effect and drug interaction detection and management, resolution of medication adherence issues, and provision of drug information). Given the variability in assessment, analysis, and presentation of outcomes in identified studies, we were unable to conduct a meta-analysis.
Results
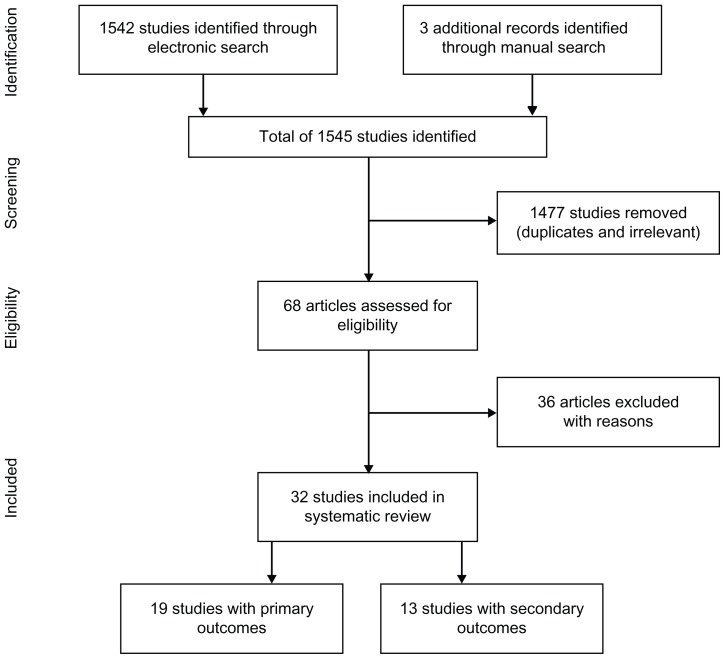
From 1545 search matches, 68 articles were assessed for eligibility and, of these, 36 were excluded because they were published in a language other than English (n = 3), were in abstract form (n = 11), were review articles (n = 3), were qualitative studies (n = 4), or were not regarding pharmacist clinical care activities or intervention (n = 15) (Figure 1). Thirty-two publications met our eligibility criteria and were included.10–41 Among these publications, 19 evaluated the primary outcomes of interest10–28 and 13 contained information on the secondary outcomes.29–41 Tables 2 and 3 summarize these studies.
Figure 1.
Selection process for study inclusion.
Table 2.
Summary of studies with primary outcomes
| Source | Country, City (State) Study start year Estimated study end* Sample size Mean age (years) % Male % MSM % BL % WH |
Study design and objectives | If examined interventions, description of intervention | Inclusion/exclusion criteria | Description of pharmacist’s role Pharmacist’s role central or peripheral to study |
Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Method of ARV adherence assessment ARV adherence outcomes |
HIV viral load (copies/mL) | CD4+ cell count (cells/mm3) | Other outcomes | ||||||
| Ostrop10 | Canada, Alberta NR NR N = 64 36** 95% NR NR NR |
Cohort study to examine ARV adherence and duration of therapy via tool usage (computer generated individualized schedule, pill box, and electronic reminder device) | Received medication and adherence counseling, monitoring, medication interventions, introduction to adherence tools. Provided individualized schedules, pill boxes, and pagers | Replied to questionnaire ≥1 time, ≥18 years, starting ≥1 new ARV. Exclude: not responsible for taking own medications, enrolled in other research, unable to complete questionnaire | Created computer-generated schedules in collaboration with patient (taking account patient needs and regimen requirement). Programmed electronic reminders using beepers Role: Peripheral |
Refill Median adherence = 95%; 75% patients had >91% adherence. No significant difference in adherence between use of schedule (92%) or pill box (89%). Adherence with beepers was 76% |
NR | NR | Tool usage: 61% used a tool at 6 and 12 months. Schedules used by 48%, pill box by 20%, pagers by 8%. ARV persistence: 74% remained on ARVs at 12 months |
| McPherson-Baker11 | USA, Miami (FL) NR NR N = 42 46 100% 14.3% 73.8% 11.9% |
Controlled before-after study to test efficacy of brief medication counseling and behavioral intervention in improving ARV adherence | Intervention: Monthly pharmacist visits x5; given pill box and adherence counseling; instructed on how to fill pill box. Control: usual care including only pharmacy overview of medications but no pill boxes. Matched on age, CD4+, risk factor, ethnicity | Nonadherent (failure to refill ARVs and OI medications or hospitalization for OI); function independently. Exclude: Karnofsky <60, has primary caregiver, living in facility, mental disability, history of clinic loss to follow-up | Educated patient on basic HIV information, impact of HIV on body, purpose of ARVs, clarification of regimen and potential toxicity. Gave pill box and taught how to fill it. At follow- up visits reviewed regimen, adherence barriers, AEs, and gave positive reinforcement Role: Central | Refill Significant increase in adherence at 5 months post-intervention (t = 4.2 1, P < 0.01). Intervention group: Baseline = 46.9% 5 months post-intervention = 75.8% Control group: Baseline = 54.4% 5 months post-intervention = 39.3% |
Mean VL from baseline to 5 months post-intervention: Intervention: 99, 213 to 81,600 c/mL. Control: 142,848 to 119,275 c/mL | Mean CD4+ from baseline to 5 months post-intervention: Intervention: 143.1 to 136.50 Control: 193.5 to 166.1 |
Significant increase in adherence to clinic appointments (P < 0.05) Significant decrease in hospitalizations (P < 0.05) |
| Mathews12 | USA, San Diego (CA) 1998 1999 N = 235 NR 86% 52% NR 52% |
Cohort study to assess prevalence and predictors of early ARV adherence using multiple indicators and to estimate effects of early adherence on subsequent VL and CD4+ | Patients referred to ARV monitoring clinic for therapy initiation or change. Conducted baseline interview then 30 days of EDM, interview at 30 days, and VL monitoring | Consenting HIV+ adults, receiving care at study clinic, referred to ARV monitoring clinic for treatment initiation or change, candidates for VL suppression to <400 c/mL | Selected regimen after PCP consult and review of prior ARV history, drug interactions, contraindications, patient preferences. Provided adherence counseling. Estimated average duration of therapeutic drug levels following dosing events Role: Peripheral |
EDM and self-report Adherence predictors: male, nonBlack, ARV naïve, fewer urgent appointments, no substance use, prior adherence, health beliefs, pharmacist prediction of high adherence, number of ARVs in regimen, high ARV knowledge, low ARV pessimism | Mean change in VL from baseline to 6 months inferior in EDM noncompleters (0.5 log10 change) vs completers (1.7 log10 change) | Predictors of CD4+ response were baseline CD4+ and prior ARV experience | EDM noncompletion was risk factor for worse VL and CD4+ outcomes |
| Smith13 | USA, Chapel Hill (NC) 1998 1999 N = 22 (A); 21 (B) NR (≥40 A: 32%; B: 33%) A: 77%; B: 81% NR NR A: 32%; B: 19% |
RCT to examine whether a self-management intervention based on feedback of adherence and principles of social-cognitive theory improves adherence |
|
≥18 years, give informed consent, starting new ARV regimen including a PI or change to a new PI-containing regimen | Educated on ARVs (ADRs, dosing, storage, interactions; gave medication grid, how to improve adherence, self-management and skills training. Gave diary to track nonadherence and events fostering it. Discussed diary notes and gave feedback by EDM outputs Role: Peripheral | EDM Adherence in A higher than B. Mean adherence by end of 12 weeks: A = 96%; B = 37%. OR of A vs B in taking ≥80% of doses per week = 7.8 (95% CI = 2.2–28.1) |
At least one VL < 400 c/mL (as-treated analysis): A = 64%; B = 38%. At least one VL < 400 c/mL (ITT analysis): A = 41%; B = 24% | NR | Adherence self-efficacy: not significant for between-or within-subject effect according to treatment group |
| Castillo14 | Canada, Vancouver 1997 2002 N = 489 (A); 98 (B); 201(C) A: 37; B: 39; C: 37** A: 86%; B: 85%; C: 70% NR NR NR |
Cohort study to compare impact of differing levels of HIV-pharmacy care on adherence and time to VL suppression (time from initiating ARVs to VL < 500 x2) | – | ≥18 years, treatment naïve when starting 2 NRTIs + PI or 2 NRTIs + NNRTI between August 1997–July 2000 |
|
Refill Highest proportion with >90% adherence in A (70.4%) vs 59.2% in B and 55.7% in C; P = 0.0001). No difference between B and C (P = 0.52) |
Highest likelihood of suppression at 12 months in A (75%) vs B (59%) and C (60%) (P = 0.001) Unadjusted RH of VL suppression, A vs B = 1.42 (95% CI: 1.09–1.84) Unadjusted RH of VL suppression, A vs C = 1.58 (95% CI: 1.30–1.92). Unadjusted RH of VL suppression, B vs C = 1.10 (95% CI: 0.82–1.48). RH of VL suppression, A vs B + C, adjusted for age, gender, physician experience, CD4+, VL, IDU = 1.42 (95% CI: 1.10–1.84) |
NR | – |
| Levy15 | Australia, Melbourne 2002 2002 N = 52 42 88% 58% NR NR |
Quasi RCT to determine the impact of an adherence education intervention on adherence | General education: on HIV and importance of adherence by pharmacist or RN. Individual session: pharmacist examined lifestyle and barriers; patient given medication planner, adherence devices, pharmacist pager number | ≥18 years, written consent, ARVs from the clinic. Exclude: those planning to interrupt treatment, changing ARVs within next 3 months, 100% adherent, or VL undetectable | Educated on HIV and adherence; examined patient lifestyle (eg, sleep, diet, work, etc); integrated medicines into patient’s life; gave medication planner and adherence devices (eg, pill boxes, alarms), and pager number Role: Central |
Self-report Missed doses in last 4 days: Pre = 1.9; Post = 1.0 (P < 0.001). 7 days: Pre = 3.0; Post = 1.8 (P < 0.001). 28 days: Pre = 7.4; Post = 4.2 (P < 0.001) |
Mean VL: Pre = 21,801 c/mL; Post = 17,587 c/mL (P = 0.39) | Mean CD4+ cell count: Pre = 382; Post = 406 (P = 0.70). Mean CD4+%: Pre = 20; Post = 19.5 (P = 0.83) | – |
| Gross16 | USA, Philadelphia (PA) 2001 2002 N = 110 NR (<50: 56% ) 98% 35% 77% NR |
Cohort study to determine if different refill mechanisms (ie, monthly pick-up at pharmacy, monthly mail order, or pharmacist-dispensed pill organizers) every 2 weeks were associated with differences in ARV refill adherence | – | Veteran’s administration patient, computerized records available, on stable ARV regimen >3 months |
|
Refill Total adherence: A = 99%; B = 80%; C = 91%. A vs B: P = 0.003 B vs C: P = 0.04 C vs A: P = 0.14 >85% adherence: A = 100%; B = 39%; C = 61% A vs B: P < 0.001 B vs C: P = 0.03 C vs A: P = 0.02 |
NR | NR | – |
| Rathbun17 | USA, Oklahoma City (OK) 2001 2003 N = 16 (A); 17 (B) A: 38; B: 38 A: 75%; B: 94% A: 63%; B: 76% A: 13%; B: 29% A: 75%; B: 65% |
RCT to examine the impact of a pharmacist operated adherence clinic on adherence to HAART and viral suppression |
|
Treatment naïve or experienced initiating >3 ARVs. Excluded: once-daily regimens, 3 NRTI regimen, salvage (resistance to >2 ARVs in regimen), in clinical trial, already followed in adherence clinic | Educated about ARV, food requirements, and AE management; monitored patient progress; used visual aids and reminder devices Role: Central |
EDM, self-report ITT EDM at week 28: A = 74%; B = 51% (P = 0.08). As-treated EDM at week 28: A = 82%; B = 57% (P = 0.05). Dose precision (took ARVs on schedule) at week 28: A = 53%; B = 31% (P = 0.05) |
Proportion with VL < 400 at week 16: A = 100%; B = 71% (P = 0.04). week 28: A = 94%; B = 65% (NSS). Proportion with VL < 50 at week 16: A = 63%; B = 35% (NSS). week 28: A = 63%; B = 53% (NSS) | Median increase in CD4+ (CD4+%) from baseline: A = 142 (5%); B = 97 (4%) | – |
| Frick18 | USA, Seattle (WA) 1997 2000 N = 152 (A); 109 (B) NR (≥40 A: 32%; B: 23%) A: 93%; B: 84% A: 56%; B: 47% A: 12%; B: 20% A: 75%; B: 57% |
Historically controlled trial to compare duration on ARVs, clinical indicators, and adherence between HIV+ patients in a multidisciplinary program (A) vs historical controls (B) from 6 months before initiation of HAART protocol) | – | HAART protocol: ≥18 years, treatment naïve, starting 1st HAART with PI or NNRTI, referred to protocol, filled 1st ARV within 365 days of starting protocol. Historical control: start 1st HAART 6 months prior to study start |
|
Refill If stopped HAART before 12 months Mean adherence: A = 82%; B = 85% (P = 0.46). If continued ARVs for 12 months A = 89%; B = 87% (NSS) |
If stopped HAART before 12 months- Mean log10 VL change: A = −1.98; B = −1.60 (P = 0.18). VL suppressed at 12 months: A = 66.7%; B = 48.8% (P = 0.11). If continued ARVs for 12 months- Mean log10 VL change: A = −3.22; B = −2.11 (P < 0.001). VL suppressed at 12 months: A = 88.3%; B = 84.6% (P = 0.55) |
If stopped HAART before 12 months- Mean CD4+ change: A = 132; B = 157 (P = 0.38). If continued ARVs for 12 months- Mean CD4 change: A = 227; B = 196 (P = 0.26) | Time on HAART: A > 360 days; B = 210 days (P = 0.02). If continued HAART at 12 months: A = 55%; B = 43% |
| Visnegarwala19 | USA, Houston (TX) 1999 2004 N = 11(A); 21(B); 22(C) A: 38; B: 40; C: 39.6** 0% 0% A: 91%; B: 76%; C: 86% NR |
Historically controlled study to compare VL from Directly Delivered Therapy (DDT) vs Adherence Coordination Services (ACS) vs Standard of Care (SOC) during intervention (at 4–8 months) vs post intervention (at 10–14 months) |
|
ARV naïve women, entering care and off ARVs for >2 years, restarting treatment with ≥2 new ARVs | ACS: Pharmacist made reminder calls for pharmacy refills and clinic appointments Role: Peripheral |
Self-report (DDT and ACS); pill count by empty bubble pack (DDT) A = 85%; B = 81% with 100% adherence |
VL < 400 during intervention: A = 85%; B = 54%; C = 36% (OR = 1.6; P = 0.003). 2-way comparison: OR (A vs C) = 10.5 (P < 0.001). OR (A vs B) = 0.4 (P = 0.08). OR (B vs C) = 2.1 (P = 0.3). VL < 400 post intervention: A = 80%; B = 54%; C = 45% (OR = 2.7; P = 0.1). 2-way comparison: OR (A vs C) = 4.8 (P = 0.03) | CD4+ change during intervention: A = 19; B = 262; C = 115 (P = 0.05). CD4+ change post intervention: A = 242; B = 153; C = 122 | Appointment keeping: A = 76%; B = 75%; C = 54%. Cost: A = $347 per person per month; B = $667 per person per month |
| March20 | USA, Los Angeles (CA) 2003 2005 N = 34 47 28% NR 26% 15% |
Before-after study to evaluate the impact of HIV drug optimization clinic (DOC) pharmacists’ interventions on VL and CD4+, rate of ADR, patients’ perception of own health status | PCPs referral if ARV nonadherence, ADRs, drug interactions, drug-resistant virus. Follow-up for 12 weeks or until discharged | ≥18 years, gave informed consent. Exclude: participation in other study that limited pharmacist activities at DOC | Pharmacist highly trained in HIV pharmacotherapy. Educated patient, added or discontinued medication, adjusted dosage due to renal/hepatic impairment or interaction, interpreted resistance tests, devised best-fit regimens that minimized insult to other diagnoses Role: Central |
– DOC referral for poor adherence: 47% |
Mean VL decrease during study period = 1.02 log10 c/mL (P < 0.004); 62% attained undetectable VL during study. 47% DOC referral for poor adherence: Mean VL decrease = 1.02 log10 c/mL (P < 0.01). 32% DOC referral for management of viral resistance: Mean VL decrease = 1.17 log10 c/ml (P < 0.004) | Mean CD4+ increase over study period = 54 (P < 0.0002); 63% attained CD4+ > 200. 47% DOC referral for poor adherence: Mean CD4+ increase = 88 cells (P < 0.01). 32% DOC referral for management of viral resistance: Mean CD4+ increase = 79 (P < 0.004) | 253 interventions: 53% HIV related; 47% primary care related; 100% accepted by physician. 45% patient education; 20% addition of medication; 20% dosage adjustment; 10% medication discontinuation; 4% resistance test results. ARV toxicity score decrease = 1 (P < 0.001) |
| Horberg21 | USA, Northern CA 1997 2004 N = 733 (A); 838 (B) A: 40.7; B: 40.3 A: 95%; B:82% A: 71%; B: 50% A: 12%; B: 26% A: 60%; B: 44% |
Ecological study to assess the association of clinical pharmacists with health outcomes (CD4+, VL, adherence) and utilization measures (number of hospital days, ED visits, office visits) among HIV+ patients | – | ≥18 year, initiating HAART with no prior record of ARVs, ≥12 months of health plan membership prior to ARVs, used KP pharmacy for filling medications |
|
Refill At 12 months: A = 81.1%; B = 74.0% (P = 0.04). At 24 months: A = 76.7%; B = 68.9% (P = 0.02) |
OR of VL < 500: at 12 months = 2.06 (P = 0.06); at 24 months = 1.31 (P = 0.53). OR NSS after adjusted for variables (demographics, regimen type, number of pills/day, provider panel size, baseline CD4+/VL, years known to be HIV+). Relative change in log10 VL in A vs B: at 12 months = −0.72 (P < 0.001); at 24 months = −0.33 (P = 0.005). Change in log10 VL statistically significant after adjusted for variables | Difference in CD4 in A vs B at HAART: initiation = 22 (P = 0.04); at 6 months = 23 (P = 0.06); at 12 months = 14 (P = 0.22); at 24 months = −2 (P = 0.90). CD4+ difference NSS after adjusted for variables | Change in office visits RR at 24 months = 0.95 (P = 0.06). Adjusted for provider panel ≤50 = 0.81 (P < 0.001); provider panel >50 = 0.98 (P = 0.49). Change in hospital days RR = 1.29 (P = 0.003). Change in ED visits RR = 0.68 (P = 0.008). |
| Hirsch22 | USA, 10 cities (CA) 2005 2006 N = 1353 (A); 5665 (B) A: 46; B: 46.7 A: 76.3%; B: 81% NR A: 29.4%; B: 25.2% A: 44.6%; B: 46.5% |
Cohort study to examine 1st year of HIV/AIDS pharmacy MTM program by comparing patient characteristics; ARV regimens, adherence, excess fills, contraindicated regimens, OI occurrence; pharmacy and medical costs in pilot (A) vs nonpilot (B) pharmacies | Participation in pilot Medi-Cal program (pharmacies providing MTM services for HIV+ patients by participating in the special CA Department of Health Care Services program). Pharmacies had to have >90% HIV+ patients, ability to provide specialized HIV services, identify patients who should receive MTM services | HIV+, Medi-Cal beneficiary, ≥18 years, enrolled 1/2004–12/2005, at least 1 ARV and 1 medical claim with HIV diagnosis in 2004 and intervention periods (2005).
|
Counseled and evaluated adherence, consulted with providers, managed ADR, tailored regimen to fit patient’s lifestyle or needs, discussed therapy, offered adherence packaging (eg, blister packs), offered refill reminders and weekly phone calls or home visits after ARV initiation, identified peer advocates, counseled when ARV under-or over-use detected Role: Peripheral | Refill 56.3% of A patients were 80%–120% adherent vs 38.1% of B patients (P < 0.001) |
NR | NR | Cost: Mean annual cost per patient was 10% higher in A vs B (P = 0.001); driven by medication use and mental health services. Regimen persistence: A = 56.8%; B = 34.2%. Contraindicated regimens identified: A = 11.6%; B = 16.6% OIs: A = 28.2%; B = 26.1% |
| Pirkle23 | Burkina Faso, Ouagadougou and Mali, Bamako 2003 2004 N = 56 38 44.6% NR NR NR |
Before-after comparison to explore whether measuring VL plus mDAART (1–2 doses/day is witnessed) can be used in resource limited settings for nonadherent individuals | 1 month of mDAART with weekly visits with pharmacist or adherence counselors. Intervention done by family, friend, or health care professional chosen by patient | Treatment experienced, on ARVs for 6 months before study, VL > 500 c/mL, agree to intervention | NR Role: Peripheral |
Self-report NR |
VL decreased by at least 1 log10 in 1/3 of mDAART group but no decrease noted in remaining 2/3 (baseline log10 VL = 4.18 log10 c/mL) | NR | 54% of mDAART group had major drug resistance before study entry |
| Krummenacher24 | Switzerland, Lausanne and Basel 2006 2008 N = 21 (A); 11 (B) A: 36; B: 38** A: 48%; B: 45% A: 24%; B: 0% A: 43%; B: 36% A: 57%; B: 64% |
Nonrandomized controlled pilot study to evaluate the feasibility of an interdisciplinary program for enhancing adherence to first and second line ARVs |
|
≥18 years, spoke French or German, outpatients started 1st or 2nd line ARV regimen within last 4 weeks or inpatients started 1st or 2nd line ARV regimen in hospital and were at point of discharge from hospital | Educated on nonadherence management and MI. Pharmacist technicians trained on how to handle EDMs Role: Central |
EDM Persistence (% of patients with treatment interruption): A = 97%; B = 81% (P = 0.03). Execution (% of days with correct ARV dosing): A = 97%; B = 95% (P = 0.04). Adherence (persistence + execution): A = 93%; B = 87% Adherence decreased faster in controls |
NR | NR | Study successfully conducted in Lausanne but Basel recruitment was stopped due to unsuccessful recruitment |
| Ma25 | USA, Vallejo (CA) 2006 2009 N = 75 49.3 78.7% NR 25.3% 60% |
Before-after comparison to investigate the changes in HIV clinical outcomes, ARV regimen complexity, and ARV adherence, 6 months before and after the clinical care activities of an HIV clinical pharmacist | – | HIV+, received pharmacist-recommended ARV modification. Exclude: not using KP pharmacies for refills, newly starting ARVs during study period | Reviewed ARV history, resistance tests, medication intolerance, comorbidities, drug interactions, laboratory abnormalities, etc, for ARV modification to treat HIV while simplifying regimens to improve adherence. Counseled on adherence and monitored progress Role: Central |
Refill Pre = 81%; Post = 89% (P = 0.003) |
% undetectable: Pre = 63%; Post = 96% (P < 0.0001) | Absolute CD4+: Pre = 462; At = 423; Post = 491 (P-value comparing At vs Post < 0.001). CD4+%: Pre = 23%; Post = 25%; (P = 0.007). | Mean pill burden (pills/day): Pre = 7.2 ± 3.9; Post = 5.4 ± 2.8 (P < 0.001). Dosing frequency (times/day): Pre = 2.0 ± 0.5; Post = 1.5 ± 0.5 (P < 0.001) |
| Hirsch26 | USA, 10 cities (CA) 2005 2008 N = 628 (A); 1606 (B) A: 47; B: 47.4 A: 66.7%; B: 71% NR A: 34.6%; B: 30.6% A: 35.7%; B: 44.8% |
Cohort study to examine HIV pharmacy MTM program over 3 years by assessing the association between use of pilot pharmacies and
|
Participation in pilot Medi-Cal program (pharmacies providing MTM services for HIV+ patients by participating in the special CA Department of Health Care Services Medi-Cal pilot program). Pharmacies had to have >90% HIV+ patients, ability to provide specialized HIV services, identify patients who should receive MTM services | HIV+, Medi-Cal beneficiary, ≥18 years, enrolled from 1/1/2004–12/31/2007, at least 1 ARV and 1 medical claim with HIV diagnosis in 2004 and 1/1/2005–12/31/2007.
|
Counseled on adherence, consulted with other providers, managed ADR, tailored regimen to fit patient’s lifestyle or needs, offered adherence packaging (eg, blister packs), offered refill reminders and weekly phone calls or home visits after ARV initiation, identified peer advocates, counseled when ARV under- or over-use detected. Had advanced training in HIV medical care Role: Peripheral |
Refill Adherence by end of 2007: A = 69.4%; B = 47.3% (P < 0.001). Factor associated with adherence: use of pilot pharmacy (OR = 2.74; 95% CI = 2.44–3.1; P < 0.001) after controlling for age, gender, race/ethnicity |
NR | NR | Cost per patient in 2007: A = $38,983; B = $38,856 (P = 0.92). Per year nonARV cost about 30%–40% higher in A vs B. Cost for inpatient services lower in A vs B. Regimen Persistence: A = 71.7%; B = 49.1%. Contraindicated regimens identified: A = 8.9%; B = 12.2%. OIs: 35% per year in A and B |
| Krummenacher27 | Switzerland, Lausanne 2004 2009 N = 104 39** 41% NR 42% 52% |
Before-after comparison to examine patients in adherence program; reasons for enrolling; adherence rate; clinical outcomes; pharmacy visits; reasons for ARV adjustments; reasons for program interruption | – | Referred to program between 8/2004–4/2008, ARVs delivered in EDMs, completed at least 2 pharmacist MIs | Trained in MI. Conducts MI based on IMB model, provided EDM, prepared adherence report (visit summary and EDM report) sent to physician Role: Central |
EDM Persistence (% with treatment interruption) = 87%. Execution (% of days with correct ARV dosing) = 88%. Adherence (persistence + execution) = 83%. Execution and adherence decreased over time |
Undetectability increased significantly at end of study vs baseline. No statistically significant difference in median viral load (c/mL) between end of study and baseline | No statistically significant difference in median CD4+ between end of study and baseline in those on ARVs throughout study | 1388 pharmacy visits over study period; 35 minutes per visit |
| Henderson28 | USA, Denver (CO) 2009 2010 N = 28 44** 79% 50% 25% 71% |
Before-after comparison to assess impact of adherence activities in a pharmacist-managed clinic by measuring proportion of those with ≥95% adherence before and after referral to the program | – | 18–75 years, on ARVs > 3 months, got medications from clinic pharmacy | Had 5 visits patient- tailored over 6 months (at referral, 2 weeks, 1 month, then every 2 months × 2). | Refill, self-report, therapeutic drug monitoring >95% adherence: 7% (pre) to 32% (post; P = 0.01). | 15% increase in proportion of patients with undetectable VL (P = 0.10) | NR | – |
| Discussed nonadherence reasons and strategies to improve it (eg, pill box, AE management, education, telephone refill reminders) Role: Central |
Proportion days covered: 60% (pre) to 81% (post; P < 0.0001) | ||||||||
Notes:
Estimated based on end of recruitment year plus maximum length of follow-up;
Median age.
Abbreviations: ADR, adverse drug reaction; AE, adverse effect; ARV, antiretroviral; BL, Black; CA, California; CI, confidence interval; c/mL, copies/mL; CO, Colorado; ED, emergency department; EDM, electronic drug monitor; HAART, highly active antiretroviral therapy; IDU, intravenous drug use; IMB, information, motivation, behavioral skills; ITT, intention to treat; KP, Kaiser Permanente; mDAART, modified directly administered antiretroviral treatment; MI, motivational interview; MSM, men who have sex with men; MTM, medication therapy management; NC, North Carolina; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NR, not reported; NSS, not statistically significant; OI, opportunistic infection; OK, Oklahoma; OR, odds ratio; PA, Pennsylvania; PCP, primary care provider; PI, protease inhibitor; PK, pharmacokinetic; RCT, randomized controlled trial; RH, relative hazard; RN, registered nurse; RR, rate ratio; TX, Texas; VL, viral load; VS, versus; WA, Washington; WH, White.
Table 3.
Summary of studies with secondary outcomes
| Source | Country, City (State) Study start year Estimated study end* Sample size Mean age (years) % Male/MSM/BL/WH |
Study design and objectives If examined intervention, description of intervention |
Inclusion/exclusion criteria | Description of pharmacist’s role Pharmacist’s role Central or peripheral to study |
Outcomes | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Number of clinical care activities of interventions conducted % Accepted by physician/team |
Majority of clinical care activities or interventions related to: | Other outcomes | |||||
| Walji29 | Canada, Vancouver 1989 1989 N = 285 NR NR/NR/NR/NR |
Descriptive study to characterize the type, frequency, and acceptance by physician or patient of pharmacist-initiated clinical care activities regarding AZT in nonhospitalized patients with AIDS Intervention: none |
NR | Interviewed patients; explained information about clinical trial, AZT, adverse effects and management; examined signs and symptoms of efficacy and toxicity using data from patient and chart; intervened if suboptimal utilization or adverse effects Role: Central |
75 clinical care activities Accepted: 97% |
NR | – |
| Geletko30 | USA, Providence (RI) 1993 1994 N = 12 (HIV+); 19 (HIV−) HIV+: 42; HIV−: 64.7 100%/NR/NR/NR |
Descriptive study to evaluate differences in pharmaceutical care between hospitalized HIV+ patients and ID consult HIV− patients Intervention: none |
NR | Monitored HIV+ and hospitalized ID consult patients. Clinical care activities included decrease/increase dose, initiate/change/discontinue drug, prevent ADR/allergy/interaction, pharmacokinetics, provide drug information Role: Central |
218 clinical care activities HIV+: 64% (97% significant to extremely significant); HIV−: 36% (55% significant to extremely significant) (NSS) Accepted: HIV+: 85%; HIV−: 86%. |
Activities performed: (for HIV+/HIV− patients) Decrease dose: 8.6%/7.6% Increase dose: 10.1%/3.8% Discontinue drug: 26.6%/24.1% Initiate treatment: 10.8%/6.3% Prevent interactions: 4.3%/1.3% Prevent ADR/allergy: 7.9%/5.1% Provide drug info: 20.9%/16.5% |
Cost avoidance = $1888.35 (mean cost-avoidance per clinical care activity = $49.69). Difference between groups with regard to expected outcome for cost-avoidance, prevention of ADR/errors, enhance treatment efficacy, knowledge gained were statistically significant |
| Bozek31 | USA, Baltimore (MD) 1995 1996 N = 32 (HIV+); 32 (HIV−) HIV+: 36; HIV−: 50 HIV+: 63%; HIV−: 44% HIV+: 6%; HIV−: NR HIV+: 91%; HIV−: 72% HIV+: 9%; HIV−: 28% |
Descriptive study to assess differences in the rate and cost of pharmacotherapeutic clinical care activities performed for HIV+ and HIV− inpatients and to compare medication use between HIV+ and HIV− patients Intervention: none | NR | Performed medical rounds, chart review, therapeutic drug monitoring. Intervened to change therapy for untreated indication, drug use without indication, subtherapeutic dose, overdose, ADR, interaction, inappropriate route Role: Central |
HIV+: 4.6 clinical care activities per patient (95% CI: 3.2–6.1); HIV−: 1.9 per patient (95% CI: 1.2–2.6) (P < 0.05) Accepted: NR |
DRP identified: 15%: drug without indication 13%: overdose 13%: ADRs 12%: improper drug 11%: subtherapeutic dosage 10%: monitoring 10%: incorrect drug route 7%: untreated indications 6%: not receive drug |
Drug acquisition cost: decreased by 14% for HIV+ and increased by <1% for HIV−. Hospital length of stay: HIV+: 11.5 days; HIV−: 7.3 days (NSS) |
| Garey32 | USA, Chicago (IL) 1998 1998 N = 60 NR NR/NR/NR/NR |
Descriptive study to portray and characterize the pharmacist’s clinical care activities in an HIV clinic. Estimate the percentage of clinical care activities accepted by physician Intervention: none |
NR | Compared inpatient orders to outpatient regimen; evaluated accuracy and appropriateness of orders; examined for dosing errors and interactions; contacted physician to clarify medication issued; intervened with house staff Role: Central |
68 clinical care activities conducted (at least 1 intervention for 70% of patients): 94% rated clinically significant Accepted: 93% |
DRP identified: 27%: interaction 20%: sub-therapeutic dosage 17%: drug omission 17%: wrong drug 14%: other problems (duplication, incorrect regimen, addition of PCP prophylaxis) 5%: overdosage |
– |
| Geletko33 | USA, Providence (RI) 1996 2000 N = 70 NR 100%/NR/27%/53% |
Descriptive study to characterize pharmaceutical-related clinical care activities in a pharmacist-directed HIV clinic Intervention: none |
NR | Evaluated adherence, made recommendations on ARV initiation, entered prescription, dispensed medications, called patient 1 week after initiation to assess adherence and tolerance Role: Central |
1365 clinical care activities documented (mean of 1.7 per patient): 89% clinically significant 62% enhanced treatment efficacy Accepted: NR |
Activities performed: 39%: medication counseling 17%: monitoring 7%: new drug therapy 6%: drug info to providers 5%: change dosage 5%: patient referral |
– |
| Segarra-Newnham34 | USA, West Palm Beach (FL) NR NR N = 51 49 NR/NR/NR/NR |
Descriptive study to describe the utility of a clinical pharmacist’s evaluation of HIV+ patients upon hospital admission Intervention: none |
HIV+, admitted for at least 24 hours | Monitored HIV+ inpatients; wrote Pharmacy Admission Note within 24 hours of admission; evaluated and communicated correct regimen; educated patients medications; made pharmacotherapy clinic appointment Role: Central |
Total of 317 clinical care activities (median 4.2 per patient); 55% of recommendations avoided medication errors via reconciliation Accepted: 88% |
Activities performed: 29%: drug information 20%: discontinue medications 16%: decrease dose 13%: restart medications 8%: pharmacokinetics 6%: increase dose 5%: nonformulary |
– |
| De Maat35 | Netherlands, Amsterdam NR NR N = 138 (A); 130 (B) 42.6** 87.7%/NR/NR/NR |
Controlled before-after comparison to evaluate the usefulness of drug-interaction interventions by clinical pharmacist. Intervention: Arm A: medication list provided to physician. Arm B: medication list + pharmacist’s drug interaction notification + how to handle it |
Scheduled outpatient visit, signed informed consent | Obtained pharmacy records and screened list of drugs for drug interactions and sent notification to physician along with advice on how to handle it Role: Central |
Arm A: 36 interventions Arm B: 33 interventions Accepted: NR |
Most common interactions involved: 58.5%: NVP 20.7%: BZD 14.6%: methadone 11%: SSRIs 8.5%: RTV 7.3%: d4T |
There was a decrease in number of interactions between baseline and 3 months but effect similar between 2 arms |
| Foisy36 | Canada, Edmonton 2002 2003 N = 57 NR 66.6%/NR/NR/21% |
Descriptive study to portray implementation of DOT to inner-city patients and the identification and management of DRPs and outcomes during 14 months of a pharmacist position Intervention: none |
NR | Obtained baseline data (medication history, illicit drug use, lab data); selected ARVs with physician; provided medication counseling and weekly patient follow-up; communicated patient progress and adherence to physician Role: Central |
149 DRP identified (mean 2.6 DRP per patient) Accepted: >95% |
DRP identified: 38%: adverse effects 21%: interactions 16%: initiate new medication for comorbidities 13%: adherence issues 7%: medication not indicated 5%: dose adjustment |
– |
| Sterling37 | USA, Lexington (KY) 2001 2003 N = 20 (pre); 51 (post) Pre: 40; post: 38 Pre: 90%; post: 76.4% NR/NR/NR |
Historically controlled trial to examine change in number of ARV errors 1 year prior to (March 2001–March 2002) and 1 year after (April 2002–March 2003) the implementation of pharmacy admission notes Intervention: none |
≥18 years, HIV diagnosis and ARV orders on admission. Exclude: HIV diagnosis during admission, length of stay <1 day, pregnant | Within 24 hours of admission, interviewed patient; conducted chart review; documented patient’s demographics, recent VL/CD4+, diagnosis, home and inpatient medications. Evaluated patient’s medications for drug interaction, verify doses and frequencies, assess prior ARV adherence, and determine need for dose adjustments Role: Central |
Pre: 1 of 27 ARV errors identified and addressed by pharmacy note. Post: 3 of 46 ARV errors detected and addressed in pharmacy admission notes. No improvement in detection of medication errors with pharmacy admission note Accepted: NR |
NR | – |
| Heelon38 | USA, Springfield (MA) 2005 2006 N = 99 (pre); 100 (post) Pre: 45; post: 45** Pre: 51%; Post: 48% NR Pre: 29%; post: 27% Pre: 18%; post: 22% |
Historically controlled trial to compare duration of ARV-related error in hospitalized patients prior to (pre: 1/2005–6/2005) and after (post: 8/2005–2/2006) presence of pharmacist’s activities Intervention: none |
≥18 years, admitted to hospital, on HAART Exclude: patients in pharmacist’s outpatient clinic | Intervene on ARV errors by discussing with staff or MD. Retrospectively identified ARV errors, including: incomplete regimen, incorrect dose, incorrect schedule, drug interaction, incorrect formulation, incorrect ARV, duplicate therapy Role: Central |
73 ARV errors in 41 patients (17% pre versus 24% post) No significant difference in frequency or type of error pre versus post Accepted: NR |
DRP identified: 45%: incomplete regimen 30%: incorrect dose 8%: incorrect schedule 7%: incorrect formulation 3%: incorrect ARV |
Length of time until error corrected significantly shorter post pharmacist (84 hours pre versus 15.5 hours post; P < 0.0001). Mean (SD) number of prescribed ARVs was 3.5 (0.8) pre versus 3.7 (0.7) in the post phases |
| Pastakia39 | US, Chapel Hill (NC) 2006 2006 N = 68 45** 71%/NR/78%/18% |
Descriptive study to evaluate frequency and severity of ARV prescribing errors in inpatients, hospitalization and discharge errors, physician acceptance of pharmacy recommendations, risk factors associated with occurrence of errors Intervention: none |
HIV+, ≥18 years, received care at ID/HIV clinic of hospital, continued ARVs upon admission | Reviewed ARVs of HIV+ inpatients, identified ARV errors, resolved errors by making recommendations to clinical team. Errors classified as: Class 1: unlikely to cause patient discomfort or clinical deterioration; Class 2: had the potential to cause moderate discomfort or clinical deterioration; Class 3: had the potential to cause severe discomfort or clinical deterioration Role: Peripheral |
Initial regimen: 72% of patients had at least 1 error; 56% had at least 1 class 2/3; inpatient physician errors made up 45% of errors (all class 2/3); inpatient pharmacy errors made up 33% of errors (37% class 2/3). Initial regimen and hospitalization: 119 errors observed (82% class 2/3); 84% of patients had at least 1 error; 65% had at least 1 class 2/3 Accepted: 100% |
NR | No factor (patient, provider, drug regimen characteristics) was predictor of initial inpatient ARV errors. Use of ATV was a predictor of errors during hospitalization/discharge. Risk factor predisposing patients to having >1 class 2 or 3 error was regimens requiring conversion from outpatient to hospital formulary |
| Horace40 | USA, Augusta (GA) 2007 2009 N = 50 (descriptive); 34 (pilot) NR NR/NR/NR/NR |
Descriptive study to retrospectively identify common medication-related problems for HIV+ patients and a historically controlled trial to evaluate the effect of a pharmacy monitoring services pilot program Intervention: none |
NR | Followed HIV+ patients; contacted patient or family or pharmacy to obtain correct information; communicated medication errors with medicine team; intervened on medication related problems Role: Central |
42 clinical care activities Accepted: 95% |
Activities performed: 35%: complete home medication list; 18%: resolve drug–drug interactions; 18%: reconciled inpatient medications to home medication list | Home medication documentation improved by 24%; matching inpatient to home medications by 26%. 9% of patients had medications discontinued for >24 hours (100% had appropriate reason) versus 42% prior to pharmacist (67% had no appropriate reasons). Appropriate ARV dosing and scheduling increased by 21% |
| Carcelero41 | Spain, Barcelona 2007 2008 N = 189 45 71%/26%/NR/NR |
Descriptive study to identify and describe ARV-related errors in medication prescribing and determine degree of acceptance of pharmacist’s patient-care activities Intervention: none |
HIV+, ≥18years, admitted to Hospital Clinic, prescribed ARVs | Checked for drug–drug interactions, incorrect or incomplete ARV regimens, omitted doses, incorrect doses, lack of dose reduction for renal/hepatic impairment, and incorrect schedule Role: Central |
247 admissions reviewed, 60 drug-related problems identified in 41 patients (21.7%) Accepted: 92% |
DRP identified: 33%: drug interaction 17%: incorrect dose 15%: dose omission 10%: omission of ARV 8.3%: prescription of alternative ARV 5%: incorrect schedule |
Factors associated with increased risk of ARV problems: renal impairment, use of ATV, admission to unit other than ID unit. Majority of errors occurred at admission |
Notes:
Estimated based on end of recruitment year plus maximum length of follow-up;
median age.
Abbreviations: ADR, adverse drug reaction; ARV, antiretroviral; ATV, atazanavir; AZT, zidovudine; BL, Black; BZD, benzodiazepine; CI, confidence interval; Cost avoidance, (acquisition cost of drug regimen at time of evaluation – acquisition cost of recommended drug regimen) × duration of treatment while patient in hospital; d4T, stavudine; DOT, directly observed therapy; DRP, drug-related problem; GA, Georgia; HAART, highly active antiretroviral therapy; ID, infectious diseases; IL, Illinois; KY, Kentucky; MA, Massachusetts; MD, Maryland; MSM, men who have sex with men; NC, North Carolina; NR, not reported; NSS, not statistically significant; NVP, nevirapine; PCP, pneumocystis carinii pneumonia; RI, Rhode Island; RR, relative risk; RTV, ritonavir; SD, standard deviation; SSRI, selective serotonin reuptake inhibitor; VL, viral load; WH, White.
Publications evaluating HIV clinical pharmacists’ impact on primary outcomes
These studies were published between 2000 and 2011 and were primarily conducted in the US (68%). Observational cohort studies (32%) and before-after comparisons (32%) were the most common study designs. Baseline sample sizes ranged from 28 to 7018 (median = 64); in studies that reported mean age, participant mean age ranged from 36 years to 49 years; and the percentage of male study participants ranged from 0% to 100% (median = 80%). The percentage of participants who were Black ranged from 15% to 83% (median = 26%; not stated in 32% of studies); the proportion of White participants ranged from 12% to 71% (median = 52%; not stated in 32% of studies); and the percentage of men who have sex with men (MSM) ranged from 0% to 70% (median = 51%; not stated in 47% of studies). The pharmacist played a central role in the study objectives of 53% of included publications11,14,15,17,20,21,24,25,27,28 and 63% of studies examined the impact of pharmacist interventions (see Methods section for definition).10–13,15,17,19,20,22–24,26
The majority of the reviewed studies examined the impact of pharmacists in HIV ambulatory care clinic setting (63%),10–12,15–21,25,28 followed by outpatient community pharmacies (26%).14,22,24,26,27 The main pharmacist role was the provision of medication adherence counseling and tools for adherence improvement (including pill boxes, refill reminders, beepers, alarms, medication schedules, blister packs, medication diaries, etc). Other pharmacist activities included patient education (regarding dosing, adverse effects, drug interactions, medication storage, missed doses, adherence, methods of improving adherence, etc); ARV regimen selection; ARV initiation, discontinuation, and dose adjustment for renal/hepatic impairment; and monitoring for ARV adverse effects and drug interactions.
ARV adherence
In the 18 studies that examined ARV adherence10–19,21–28 (adherence not assessed in March et al20), the most common method of adherence assessment was based on medication refill records (56%), followed by patient self-report (33%), and electronic drug monitoring using medication event monitoring systems (MEMS®, 28%). Other less frequently used methods included pill count and therapeutic drug level monitoring. Approximately 78% of studies used only one adherence assessment method and 17% used two methods.
Among the 10 publications in which the pharmacist’s role was central,11,14,15,17,20,21,24,25,27,28 adherence was compared between the pharmacist group versus a control group in eight studies;11,14,15,17,21,25,27,28 all of which found an association between assignment to the pharmacist group and improved adherence outcomes. Nine studies examined interventions or clinical care activities where the pharmacist had a peripheral role,10,12,13,16,18,19,22,23,26 among which five reported medication adherence outcomes by comparing the pharmacist group versus a control group.13,16,18,22,26 Four of these studies reported a positive association between adherence and allocation to the pharmacist group13,16,22,26 and one showed no statistically significant difference between the two arms.18
Among 13 studies that compared adherence outcomes of a pharmacist-engaged study arm versus a control arm,11,13–18,21,22,24–26,28 nine reported percent ARV adherence as a continuous outcome in each group at the end of the follow-up period.11,13,16–18,21,24–26 In these studies, adherence in the pharmacist arm was 2%–59% (median = 19%) higher as compared to the control arm. Four studies used other methods of comparison to present the impact of pharmacist care on adherence.14,15,22,28 Castillo et al,14 found that 14.7% more patients who obtained service from AIDS tertiary care hospital pharmacies had >90% adherence compared to those with no pharmacist contact. Hirsch et al,22 found that 18.2% more patients receiving ARVs from pilot Medi-Cal pharmacies, featuring pharmacists with HIV training, had an adherence of 80%–120% in comparison to those not enrolled in this program. In a study by Henderson et al,28 25% more patients had >95% adherence after referral to the pharmacist-managed clinic versus prior to referral. Lastly, Levy et al15 reported that participants missed 1.2 fewer doses in the past 7 days after receipt of a pharmacist-provided adherence education session versus the period of observation prior to this intervention.
HIV viral load
Among the ten studies that assessed the central role of the pharmacist,11,14,15,17,20,21,24,25,27,28 nine examined viral load outcomes.11,14,15,17,20,21,25,27,28 In six of these studies, pharmacist involvement was associated with clinically or statistically significant viral load reductions or a greater proportion of maximal viral suppression,14,17,20,21,25,28 while in three, no association with pharmacist care was observed.11,15,27 The pharmacist assumed a peripheral role in nine studies,10,12,13,16,18,19,22,23,26 among which five reported virologic outcomes.12,13,18,19,23 In four of these studies, a favorable association was noted between viral load reduction and allocation to the pharmacist-involved study arm,12,13,18,23 whereas no relationship between virologic response and pharmacist care was reported by one study.19
CD4+ cell count
In the ten studies where a pharmacist played a central role, seven also assessed immunologic outcomes.11,15,17,20,21,25,27 Among these studies, two revealed an increase in CD4+ cell count related to receipt of pharmacist care20,25 and five showed no association.11,15,17,21,27 Of the nine studies in which the pharmacist had a peripheral role, only two reported immunologic outcomes18,19 and in both no relationship was seen between the pharmacist arm versus the control arm.
Other outcomes
Among the ten studies investigating the pharmacist’s central role, several reported other favorable outcomes, including an increase in adherence to clinic appointments and reductions in variables such as hospitalizations,11 ARV toxicity scores,20 physician office visits, number of hospital days, emergency department visits,21 pill burden, and daily dosing frequency.25 Other outcomes in the nine studies where the pharmacist assumed a peripheral role included no changes in variables such as ARV adherence self-efficacy,13 retention on ARV at 12 months,18 and frequency of incident opportunistic infections.22,26 However, there were increases in the time on ARV therapy,18 improved appointment keeping,19 higher likelihood of remaining on ARV,22,26 fewer contraindicated ARV regimens,22,26 and a higher cost in the study arm involving the pharmacist.19,22
Publications evaluating HIV clinical pharmacists’ impact on secondary outcomes
These studies were published between 1992 and 2011 and 69% were conducted in the US. Approximately 80% of these studies were descriptive in nature. Baseline sample sizes ranged from 31 to 285 (median = 70); in studies reporting mean age, participant mean age ranged from 36 years to 65 years (age not stated in 38% of studies); percentage of male study participants ranged from 49% to 100% (median = 71%; not stated in 31% of studies). The percentage of participants who were Black ranged from 27% to 82% (median = 53%; not stated in 69% of studies); proportion of participants who were White ranged from 18% to 53% (median = 20%; not stated in 62% of studies); and percentage of MSM ranged from 6% to 26% (not stated in 85% of studies).
In 92% of these studies, the central role of a pharmacist was evaluated29–38,40,41 and approximately 85%–100% of the pharmacists’ suggestions were accepted by the physician or health care team. Sixty-nine percent of studies examined pharmacists’ impact in the inpatient medical center setting30–32,34,37–41 and 23% assessed this role in the outpatient ambulatory care clinics.29,33,35 The clinical care activities performed by pharmacists in these reports included adjustments in drug doses, medication initiation/discontinuation, monitoring and prevention of drug interactions or adverse drug reactions, and the provision of drug information and medication counseling.
In one study, the researchers noted an improvement in the inpatient documentation of outpatient medications, a reduction in inappropriate discontinuation of outpatient medications, and an increase in ARV prescription accuracy for inpatients.40 Another study examined the benefits of pharmacists on the inpatient service and reported a substantial reduction in the length of time taken to correct an ARV error.38 Conversely, in the only study that examined the effect of a pharmacist’s interventions (see Methods for definition), the reduction in the number of drug interactions between patients whose physician received only their medication list was no different from those whose physician received both the medication list and the pharmacist’s drug interaction notification and management suggestions.35
Discussion
In this systematic review, we evaluated the impact of HIV pharmacists on HIV clinical outcomes, health utilization measures, ARV modifications, and other descriptive variables. In all but one study,18 the involvement of an HIV pharmacist in patient care was associated with clinically and statistically significant improvements in ARV adherence. The majority of reviewed studies also indicated that HIV pharmacist’s care was associated with greater viral load suppression. Evidence of any influence of pharmacists on immunologic outcomes was unclear and attenuated, which may have been due to lack of reporting of CD4+ cell count in many studies, insufficient duration of follow-up to observe substantial changes, the lack of an effect, or the more erratic nature of this outcome measure.
Several study-related factors limited the depth of our review. The most crucial limitation of several studies was the lack of reporting and/or adjustment for baseline demographics and confounders. The absence of reporting of clinical outcomes data in many studies and methodological constraints, such as reporting adherence as dichotomous or categorical variables or other methods, precluded a meta-analysis. Other common limitations included small sample size, short duration of study follow-up, incomplete description of the pharmacist’s role or the complexity of multicomponent interventions, and the use of unconventional methods of adherence calculation. Lastly, as with any systematic review, there is the potential for positive publication bias influencing the aggregate results.
The reviewed studies provide a broad spectrum of HIV pharmacist activities. It is noteworthy that the majority of the reviewed studies were conducted in HIV ambulatory care or inpatient medical center settings. HIV pharmacists practicing in community pharmacies are increasingly called upon to provide ARV adherence training, patient education, and drug information, yet outcome data from such activities are not well-represented in the literature. This may be due to the under-recognized value of these services or the challenges associated with gaining combined access to laboratory medical record and community pharmacy data.
We found a plethora of descriptive studies on ARV-related errors identified and resolved by the pharmacist and the degree of acceptance of pharmacist-related activities, as well as observational studies on the consistent evidence of a positive impact of HIV clinical pharmacists on ARV adherence. Therefore, future mixed methods research, including qualitative and quantitative studies should examine the pharmacist–patient relationship, focus on determining crucial pharmacist functions which have the most impact on adherence, and test these findings in randomized controlled trials with large sample sizes. Additionally, studies should examine cost-effectiveness of pharmacists (including cost savings associated with improvements in clinical markers, as well as other outcomes, such as reductions in extraneous physician visits, emergency room visits, length of hospitalization, medication errors, etc). Further research should also expand to include HIV pharmacist responsibilities that are beyond the “traditional” functions (ie, assessment of ARV accuracy, identification of drug interactions, adherence counseling, patient/provider education, etc). These roles may include the involvement of pharmacists in conducting clinical trials, performance of motivational interviewing, interpretation of drug resistance tests and prescription of ARVs, methods of tailoring adherence-enhancing tools based on individual reasons for nonadherence, and impact on HIV prevention (eg, through offering pre- or post-exposure prophylaxis).
It is evident in this review that research on the impact of pharmacists in HIV clinical care has evolved since the first reports in 1992. This progression includes the use of more sophisticated study designs and more complex research questions. Continued research on HIV pharmacists’ impact on the clinical care of HIV-positive individuals is underway. In ClinicalTrials.gov and the US National Institute of Health Research Portfolio Online Reporting Tools database there are currently several ongoing studies examining the role of pharmacists in HIV clinical care. Four of these studies pertain to HIV prevention by assessing and expanding the pharmacist’s role in services related to intravenous drug users purchasing syringes.42–45 Another project is assessing factors related to the receipt of pharmacist-provided adherence counseling and the impact of a counseling session based on the information–motivation–behavioral skills model46,47 on HIV treatment outcomes.49 A randomized controlled trial is examining the impact of pharmacist care on ARV adherence.49 Lastly, economic outcomes of an intervention comparing methods of offering pharmacist services are also under study.50
Conclusion
In conclusion, this systematic review provides support for the positive association between HIV pharmacist activities and improvements in ARV adherence and viral load suppression. HIV pharmacist functions were related to reductions in hospitalization, physician office visits, number of hospital days, visits to the emergency department, pill burden, and inappropriate discontinuation of outpatient medications; as well as improvements in inpatient documentation of home medications and accuracy of ARV dosing. A high percentage of pharmacists’ recommendations were accepted by the physician or the health care team and the majority of the pharmacist’s functions involved ARV dosing, detection of drug interactions or adverse drug reactions, provision of drug information, ARV adherence counseling, and instructing on the use of adherence-enhancing tools. This systematic review provides further evidence that, with the growing number of HIV-positive individuals worldwide, the increasing intricacies of HIV treatment options, and the shortage of physicians in resource limited settings, clinical pharmacists trained in HIV pharmacotherapy are invaluable resources and are essential members of the HIV multidisciplinary care team.
Acknowledgments
The authors thank Gloria Won for her assistance with the search strategies, conducting the electronic search, and creating our preliminary EndNote library. The project described was supported by NIH award numbers F32MH086323, K23MH087218, and K24MH087220. Jennifer Cocohoba received a one-time investigator initiated research grant from Gilead Sciences in 2009.
Footnotes
Disclosure
The authors report no conflicts of interests in this work.
References
- 1.Centers for Disease Control (CDC) Pneumocystis pneumonia – Los Angeles. MMWR Morb Mortal Wkly Rep. 1981;30(21):250–252. [PubMed] [Google Scholar]
- 2.Corelli RL, Guglielmo BJ, Kapusnik-Uner JE, McMaster JR, Greenblatt RM. Medication usage patterns in patients with human immunodeficiency virus infection: a comparison of patient-reported medication usage with medical chart review. DICP. 1991;25(12):1374–1378. doi: 10.1177/106002809102501217. [DOI] [PubMed] [Google Scholar]
- 3.Crawford NS. Organizing pharmacists to help fight AIDS. Am Pharm. 1991;NS31(3):44–47. doi: 10.1016/s0160-3450(15)31370-2. [DOI] [PubMed] [Google Scholar]
- 4.McKnight PT, Visconti JA, Gower RE, Para MF. Zidovudine: counseling strategies and Compliance. Am Pharm. 1991;31(10):38–43. doi: 10.1016/s0160-3450(15)31350-7. [DOI] [PubMed] [Google Scholar]
- 5.Weingarten CM, Freeland B. Pharmacist participation on an HIV resource team. Am J Health Syst Pharm. 1995;52(12):1282–1284. doi: 10.1093/ajhp/52.12.1282. [DOI] [PubMed] [Google Scholar]
- 6.Foisy MM, Tseng A, Blaikie N. Pharmacists’ provision of continuity of care to patients with human immunodeficiency virus infection. Am J Health Syst Pharm. 1996;53(9):1013–1017. doi: 10.1093/ajhp/53.9.1013. [DOI] [PubMed] [Google Scholar]
- 7.Horberg MA, Hurley LB, Towner WJ, et al. WITHDRAWN: Determination of optimized multidisciplinary care team for maximal antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2012 Jan 30; doi: 10.1097/QAI.0b013e31824bd605. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lefebvre C, Manheimer E, Glanville J. Searching for studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011) The Cochrane Collaboration; 2011. [Google Scholar]
- 9.Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Including non-randomized studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.1) The Cochrane Collaboration; 2008. [Google Scholar]
- 10.Ostrop NJ, Gill MJ. Antiretroviral medication adherence and persistence with respect to adherence tool usage. AIDS Patient Care STDs. 2000;14(7):351–358. doi: 10.1089/108729100413220. [DOI] [PubMed] [Google Scholar]
- 11.McPherson-Baker S, Malow RM, Penedo F, Jones DL, Schneiderman N, Klimas NG. Enhancing adherence to combination antiretroviral therapy in non-adherent HIV-positive men. AIDS Care. 2000;12(4):399–404. doi: 10.1080/09540120050123792. [DOI] [PubMed] [Google Scholar]
- 12.Mathews WC, Mar-Tang M, Ballard C, et al. Prevalence, predictors, and outcomes of early adherence after starting or changing antiretroviral therapy. AIDS Patient Care STDs. 2002;16(4):157–172. doi: 10.1089/10872910252930867. [DOI] [PubMed] [Google Scholar]
- 13.Smith SR, Rublein JC, Marcus C, Brock TP, Chesney MA. A medication self-management program to improve adherence to HIV therapy regimens. Patient Educ Couns. 2003;50(2):187–199. doi: 10.1016/s0738-3991(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 14.Castillo E, Palepu A, Beardsell A, et al. Outpatient pharmacy care and HIV viral load response among patients on HAART. AIDS Care. 2004;16(4):446–457. doi: 10.1080/09540120410001683385. [DOI] [PubMed] [Google Scholar]
- 15.Levy RW, Rayner CR, Fairley CK, et al. for Melbourne Adherence Group. Multidisciplinary HIV adherence intervention: a randomized study. AIDS Patient Care STDs. 2004;18(12):728–735. doi: 10.1089/apc.2004.18.728. [DOI] [PubMed] [Google Scholar]
- 16.Gross R, Zhang Y, Grossberg R. Medication refill logistics and refill adherence in HIV. Pharmaco epidemiol Drug Saf. 2005;14(11):789–793. doi: 10.1002/pds.1109. [DOI] [PubMed] [Google Scholar]
- 17.Rathbun RC, Farmer KC, Stephens JR, Lockhart SM. Impact of an adherence clinic on behavioral outcomes and virologic response in treatment of HIV infection: a prospective, randomized, controlled pilot study. Clin Ther. 2005;27(2):199–209. doi: 10.1016/j.clinthera.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Frick P, Tapia K, Grant P, Novotny M, Kerzee J. The effect of a multidisciplinary program on HAART adherence. AIDS Patient Care STDs. 2006;20(7):511–524. doi: 10.1089/apc.2006.20.511. [DOI] [PubMed] [Google Scholar]
- 19.Visnegarwala F, Rodriguez-Barradass MC, Graviss EA, Caprio M, Nykyforchyn M, Laufman L. Community outreach with weekly delivery of anti-retroviral drugs compared to cognitive-behavioural health care team-based approach to improve adherence among indigent women newly starting HAART. AIDS Care. 2006;18(4):332–338. doi: 10.1080/09540120500162155. [DOI] [PubMed] [Google Scholar]
- 20.March K, Mak M, Louie SG. Effects of pharmacists’ interventions on patient outcomes in an HIV primary care clinic. Am J Health Syst Pharm. 2007;64(24):2574–2578. doi: 10.2146/ajhp070048. [DOI] [PubMed] [Google Scholar]
- 21.Horberg MA, Hurley LB, Silverberg MJ, Kinsman CJ, Quesenberry CP. Effect of clinical pharmacists on utilization of and clinical response to antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(5):531–539. doi: 10.1097/QAI.0b013e318031d7cd. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch JD, Rosenquist A, Best BM, Miller TA, Gilmer TP. Evaluation of the first year of a pilot program in community pharmacy: HIV/AIDS medication therapy management for Medi-Cal beneficiaries. J Manag Care Pharm. 2009;15(1):32–41. doi: 10.18553/jmcp.2009.15.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirkle CM, Boileau C, Nguyen VK, et al. Impact of a modified directly administered antiretroviral treatment intervention on virological outcome in HIV-infected patients treated in Burkina Faso and Mali. HIV Med. 2009;10(3):152–156. doi: 10.1111/j.1468-1293.2008.00664.x. [DOI] [PubMed] [Google Scholar]
- 24.Krummenacher I, Cavassini M, Bugnon O, Spirig R, Schneider MP for Swiss HIV Cohort Study. Antiretroviral adherence program in HIV patients: a feasibility study in the Swiss HIV Cohort Study. Pharm World Sci. 2010;32(6):776–786. doi: 10.1007/s11096-010-9437-2. Epub September 23, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Ma A, Chen DM, Chau FM, Saberi P. Improving adherence and clinical outcomes through an HIV pharmacist’s interventions. AIDS Care. 2010;22(10):1189–1194. doi: 10.1080/09540121003668102. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch JD, Gonzales M, Rosenquist A, Miller TA, Gilmer TP, Best BM. Antiretroviral therapy adherence, medication use, and health care costs during 3 years of a community pharmacy medication therapy management program for Medi-Cal beneficiaries with HIV/AIDS. Journal Manag Care Pharm. 2011;17(3):213–223. doi: 10.18553/jmcp.2011.17.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krummenacher I, Cavassini M, Bugnon O, Schneider MP. An interdisciplinary HIV-adherence program combining motivational interviewing and electronic antiretroviral drug monitoring. AIDS Care. 2011;23(5):550–561. doi: 10.1080/09540121.2010.525613. [DOI] [PubMed] [Google Scholar]
- 28.Henderson KC, Hindman J, Johnson SC, Valuck RJ, Kiser JJ. Assessing the effectiveness of pharmacy-based adherence interventions on antiretroviral adherence in persons with HIV. AIDS Patient Care STDs. 2011;25(4):221–228. doi: 10.1089/apc.2010.0324. Epub Feburary 16, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Walji N, Beardsell A, Brown G. Pharmacists’ activities in monitoring zidovudine therapy in an AIDS clinic. Can J Hosp Pharm. 1992;45(1):29–32. [PubMed] [Google Scholar]
- 30.Geletko SM, Segarra M, Copeland DA, Teague AC. Pharmaceutical care for hospitalized HIV-infected patients compared to infectious diseases consult patients without HIV infection. BMJ Pharmacotherapy. 1996;2(1):45–57. [Google Scholar]
- 31.Bozek PS, Perdue BE, Bar-Din M, Weidle PJ. Effect of pharmacist interventions on medication use and cost in hospitalized patients with or without HIV infection. Am J Health Syst Pharm. 1998;55(11):1151–1155. doi: 10.1093/ajhp/55.11.1151. [DOI] [PubMed] [Google Scholar]
- 32.Garey KW, Teichner P. Pharmacist intervention program for hospitalized patients with HIV infection. Am J Health Syst Pharm. 2000;57(24):2283–2284. doi: 10.1093/ajhp/57.24.2283. [DOI] [PubMed] [Google Scholar]
- 33.Geletko SM, Poulakos MN. Pharmaceutical services in an HIV clinic. Am J Health Syst Pharm. 2002;59(8):709–713. doi: 10.1093/ajhp/59.8.709. [DOI] [PubMed] [Google Scholar]
- 34.Segarra-Newnham M. Preventing medication errors with a pharmacy admission note for HIV-positive patients. Hosp Pharm. 2002;37(1):34–37. [Google Scholar]
- 35.De Maat MMR, De Boer A, Koks CHW, et al. Evaluation of clinical pharmacist interventions on drug interactions in outpatient pharmaceutical HIV-care. J Clin Pharm Ther. 2004;29(2):121–130. doi: 10.1111/j.1365-2710.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- 36.Foisy MM, Akai PS. Pharmaceutical care for HIV patients on directly observed therapy. Ann Pharmacother. 2004;38(4):550–556. doi: 10.1345/aph.1D444. Epub Feburary 27, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Sterling ES, Romanelli F, Martin CA, Hoven AD, Smith KM. Impact of a pharmacy-initiated HIV admission note on medication errors within an academic hospital. Hosp Pharm. 2005;40(10):874–881. [Google Scholar]
- 38.Heelon M, Skiest D, Tereso G, et al. Effect of a clinical pharmacist’s interventions on duration of antiretroviral-related errors in hospitalized patients. Am J Health Syst Pharm. 2007;64(19):2064–2068. doi: 10.2146/ajhp070072. [DOI] [PubMed] [Google Scholar]
- 39.Pastakia SD, Corbett AH, Raasch RH, Napravnik S, Correll TA. Frequency of HIV-related medication errors and associated risk factors in hospitalized patients. Ann Pharmacother. 2008;42(4):491–497. doi: 10.1345/aph.1K547. [DOI] [PubMed] [Google Scholar]
- 40.Horace A, Philips M. Identification and prevention of antiretroviral medication errors at an academic medical center. Hosp Pharm. 2010;45(12):927–933. [Google Scholar]
- 41.Carcelero E, Tuset M, Martin M, et al. interventions by the clinical pharmacist in hospitalized HIV-infected patients. HIV Med. 2011;12(8):494–499. doi: 10.1111/j.1468–1293.2011.00915.x. Epub March 13, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Case PL. Feasibility of pharmacy-based HIV intervention among IDUs: 2 New England cities. National Institutes of Health Project Reporter (project number: R21DA025010) 2010. Available from: BioMedLib.com.
- 43.Fuller CM. Pharmacy referral intervention: IDU access to services. National Institutes of Health Project Reporter (project number: R01DA022144) 2010. Available from: BioMedLib.com.
- 44.Latkin CA. Feasibility of pharmacy-based HIV interventions among IDUs: India. National Institutes of Health Project Reporter (project number: R21DA024971) 2010. Available from: BioMedLib.com.
- 45.Pielemeier N. Feasibility of pharmacy-based HIV interventions among IDUs: Ha Giang, Vietnam. National Institutes of Health Project Reporter (project number: R21DA024986) 2010. Available from: BioMedLib.com.
- 46.Fisher JD, Fisher WA, Misovich SJ, Kimble DL, Malloy TE. Changing AIDS risk behavior: effects of an intervention emphasizing AIDS risk reduction information, motivation, and behavioral skills in a college student population. Health Psychol. 1996;15(2):114–123. doi: 10.1037//0278-6133.15.2.114. [DOI] [PubMed] [Google Scholar]
- 47.Amico KR, Toro-Alfonso J, Fisher JD. An empirical test of the information, motivation and behavioral skills model of antiretroviral therapy adherence. AIDS Care. 2005;17(6):661–673. doi: 10.1080/09540120500038058. [DOI] [PubMed] [Google Scholar]
- 48.Cocohoba JM. Beyond pill-counting: effect of pharmacist counseling on antiretroviral adherence. National Institutes of Health Project Reporter (project number: K23MH087218) 2011. Available from: BioMedLib.com.
- 49.Hospital de Clinicas de Porto Alegre; Federal University of Rio Grande do Sul. ClinicalTrialsgov [website on the internet] Bethesda, MD: US National Library of Medicine; 2009. Evaluation of effectiveness of pharmaceutical care on the adherence of HIV-positive patients to antiretroviral therapy (PC-HIV) Available from: clinicaltrials.gov/ct2/show/NCT00959361. NLM identifier: NCT00959361. [Google Scholar]
- 50.Wilson IB. Nudging doctors to collaborate with pharmacists to improve medication adherence. National Institutes of Health Project Reporter (project number: RC4AG039072) 2010. Available from: BioMedLib.com.