Abstract
Purpose
The relationship between antiCD20 therapy with rituximab and the lymphocytes phenotype in patients with rheumatoid arthritis was investigated, with an attempt to establish a relationship between commonly used clinical activity indices and variations in leukocyte count, in particular natural killer (NK) lymphocytes.
Methods
Patients with seropositive (cyclic citrullinated peptides and rheumatoid factor positive) rheumatoid arthritis (according to the American College of Rheumatology 1987 criteria) refractory to conventional and antitumor necrosis factor-alpha agents who were subsequently treated with rituximab, a chimeric monoclonal antibody directed against CD20, were enrolled between January 2009 and September 2009. All subjects were treated with rituximab standard rheumatologic dose of 1.0 g on days 1 and 15 every 6 months for at least 2 years. A clinical evaluation was performed at baseline and subsequently every 3 months thereafter. At each assessment activated NK (CD56+/CD16+/CD54bright) cell count was collected and disease activity was assessed using Disease Activity Score in 28 Joints and the Simplified Disease Activity Index (SDAI).
Results
Thirty-four patients were enrolled (mean age ± standard deviation: 54.8 ± 12.8 years). Basal SDAI was 21.75 ± 5.4 and NK cell count mean value was 157.6 ± 90. After 24 months, SDAI was 14 ± 1.2 and NK cell count mean value was 301.7 ± 21 (P < 0.05). An inverted correlation between SDAI and NK count was observed at 3 months (r = −0.36, P < 0.05), 6 months (r = −0.48, P < 0.45), 9 months (r = −0.47, P < 0.05), 12 months (r = −0.41, P < 0.01), 15 months (r = −0.58, P < 0.05), 18 months (r = −0.53, P < 0.05), 21 months (r = −0.68, P < 0.05), and 24 months (r = −0.61, P < 0.05). A linear regression model between all variables collected and SDAI/Disease Activity Score in 28 Joints at 6 months and 12 months confirmed a significant relationship between SDAI/Disease Activity Score in 28 Joints and NK cell count.
Conclusion
The data confirm the clinical efficacy of rituximab and suggests the use of NK cells as a predictor of clinical response in patients with rheumatoid arthritis.
Keywords: NK cells, rituximab, predictor, response, rheumatoid arthritis
Introduction
B cells have been implicated in the pathogenesis of rheumatoid arthritis (RA) since the discovery of circulating rheumatoid factor autoantibodies in RA patients. The successful introduction of B cell-depleting monoclonal antibodies as a treatment for patients with refractory RA confirmed the important role of B cells in RA.1 Rituximab is a chimeric monoclonal antibody directed against the B cell-specific membrane protein CD20, which is used in the treatment of B cell malignancies and was approved for use in the treatment of patients with RA. The exact mechanism of influence in the immune responses in autoimmune disorders leading to clinical remission is not yet known. B cell depletion and inhibition of antibody production are unlikely to explain all of its therapeutic effects.2–4 RA has a poorly understood pathogenesis, but the widely accepted view identifies rheumatoid factor as an epiphenomenon rather than an autoantibody directly implicated in disease. Furthermore, there is not a clear association between clinical improvement after rituximab therapy and B cell depletion, rheumatoid factor titer in RA, or lowering titers of anti-double-stranded DNA antibodies for lupus patients and, thus, research in rituximab therapy in autoimmune diseases includes seeking new biomarkers that may predict or measure therapeutic response.5,6
Natural killer (NK) cells are recognized by their cytotoxic and regulatory effects, implicated in prevention of autoimmunity. For example, NK cell frequency in peripheral blood is lower in patients with lupus when compared to healthy subjects and decreased values of NK cell counts are associated with higher levels of anti-double-stranded DNA. T CD4+/CD25high cells are important natural modulators of self-antigen T cell-mediated responses. While such natural regulatory T cells are reduced in patients with systemic lupus erythematosus, inducible CD4+/interleukin 10+ regulatory T cell count increases in systemic lupus erythematosus patients probably as result of a compensatory mechanism.7–12 To date, the literature is lacking a clear, clinically useful, early predictor of clinical response.13–17 In order to gain more insight into the clinical effects of B cell depletion in RA, the effects of antiCD20-mediated B cell depletion in the peripheral blood were investigated, with a focus on a possible relationship between NK activated cells (defined as CD56+/CD16+/ CD54 bright cells) and clinical response in terms of Disease Activity Score in 28 Joints (DAS28) and Simplified Disease Activity Index (SDAI).18
Methods
Patients and study design
All patients (enrolled between January 2009 and September 2009) were diagnosed according to the 1987 American College of Rheumatology (formerly, the American Rheumatism Association) criteria for RA19 and were positive for both rheumatoid factor and anti-cyclic citrullinated peptides (CCP). All patients had RA refractory to standard antitumor necrosis factor drugs, and so were treated with rituximab every 6 months at standard rheumatologic dose of two 1000 mg intravenous infusions, separated by 2 weeks with 100 mg intravenous methylprednisolone or equivalent before infusions, and accompanied by weekly 15 mg subcutaneous methotrexate.
Disease activity
Disease activity was assessed using DAS28 and the composite index SDAI. SDAI is the numerical sum of five outcome parameters: tender and swollen joint counts (based on a 28-joint assessment), patient and physician global assessment of disease activity (visual analogue scale 0–100 mm), and level of C-reactive protein (normal <1 mg/dL). The cut-off values for disease activity states are: remission ≤5, low disease activity ≤20, moderate disease activity ≤40, and high disease activity >40. Calculations of DAS28 were based on the following: number of swollen and tender joints (employing the 28-joint count), evaluator’s and/or patient’s global assessment of disease activity, C-reactive protein, or erythrocyte sedimentation rate. The following formula is the basis for the calculation:
TJC and SJC represent tender and swollen joint count, respectively, ESR stands for erythrocyte sedimentation rate (mm/hour), and GH is patient’s general health, ie, assessment of disease activity measured on a visual analog scale of 100 mm.
Flow cytometric analysis
Fresh blood was collected by venipuncture in ethylenediaminetetraacetic acid-coated vials. Immunophenotyping was performed with the following monoclonal antibodies conjugated with fluorescein isothiocyanate, phycoerythrin, or phycoerythrin cyanine 5: CD54, CD16, CD56 (Immunotech, Marseille, France). The isotype-matched negative control used was mouse immunoglobulin G1 conjugated with fluorescein isothiocyanate, phycoerythrin, or cyanine (MOPC-21) monoclonal antibodies (BD Pharmingen, Milan, Italy). Fluorescence was measured using a FACScan™ flow cytometer (BD Biosciences, San Jose, CA). Briefly, 100 μL of blood was labeled with 10 μL monoclonal antibodies for 20 minutes in the dark. Red blood cells were removed by incubating the samples with lysing solution for 10 minutes in the dark. Tubes were centrifuged using a Hettich Zentrifugen EBA 21 (Tuttlingen, Germany) for 5 minutes at 1200 rpm. Samples were treated further with washing solution and were centrifuged for 5 minutes at 1200 rpm. Finally, cells were suspended in cell fixation solution and were ready for flow cytometry measurement. Lymphocytes were gated based on their forward and side-scatter characteristics. Information regarding the percentage of peripheral NK cells CD (CD56+/CD16+) expression was obtained. As reported by Bowles et al,20 quantification of the number of NK cells with bright expression of CD54 is a reproducible marker for NK activation induced by monoclonal antibody-coated tumor cells. The number of CD54bright NK cells was therefore evaluated as a measure of NK cell activation.
Statistical analysis
Pairwise comparisons were based on the Wilcoxon matched-pairs signed-rank test. Correlation between variables was evaluated with Spearman’s rho.
All values of P < 0.05 were considered to indicate statistical significance (two-tailed test). Linear regression models were estimated using all variables collected as covariates and SDAI and DAS28 as the dependent variable.
Results
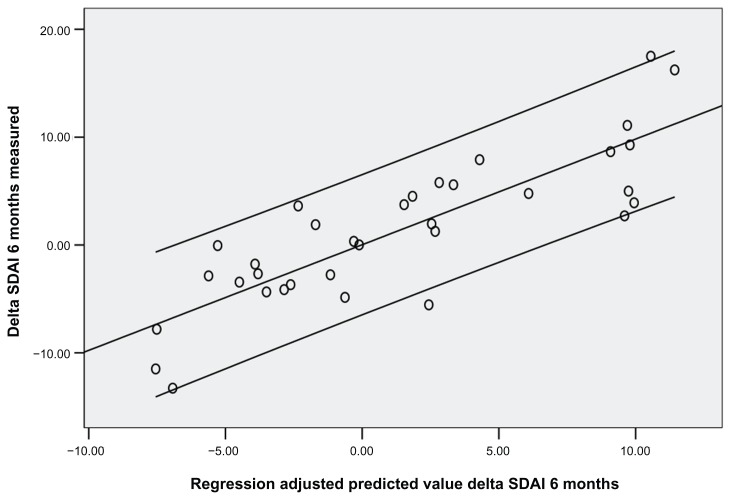
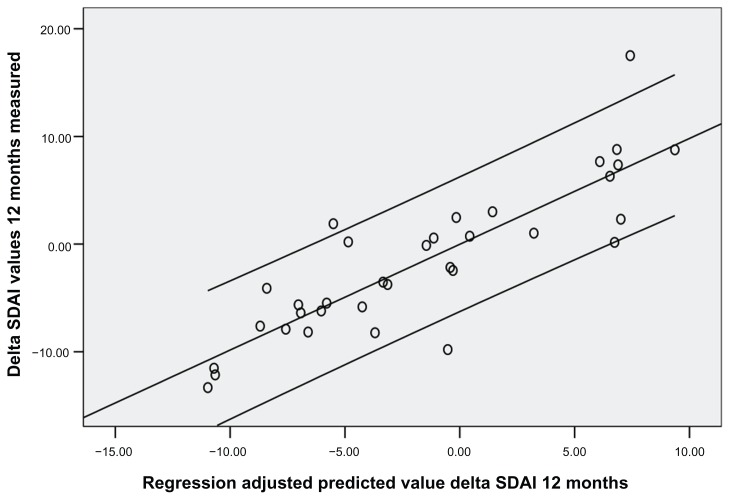
Thirty-four patients were enrolled (mean age ± standard deviation was 54.8 ± 12.8 years; 22 females, 12 males). Disease duration at the first course of rituximab was 5.8 years. Rheumatoid factor mean values were 115 ± 25 at baseline, 53 ± 40 after 1 year, and 47 ± 20 after 2 years (P < 0.05). CCP mean values were 86 ± 30 at baseline, 34 ± 25 after 1 year, and 39 ± 30 after 2 years (P < 0.05). DAS28 was 5.25 ± 1.3 at baseline, 4.47 ± 0.7 at 1 year, and 3.34 ± 1.1 after 2 years (P < 0.05). Basal SDAI was 31.75 ± 5.4 and NK cell count mean value was 157.6 ± 90. After 1 year SDAI was 18.3 ± 20.2 and NK cell count was 221 ± 90 (P < 0.05). After 24 months SDAI was 14 ± 1.2 and NK cell count was 301.7 ± 21 (P < 0.05). SDAI, DAS28, and NK cell count were assessed every 3 months. An inverse correlation between SDAI and NK cell count was observed at 3 months (r = −0.36, P < 0.05), 6 months (r = −0.48, P < 0.05), 9 months (r = −0.47, P < 0.05), 12 months (r = −0.41, P < 0.01), 15 months (r = −0.58, P < 0.05), 18 months (r = −0.53, P < 0.05), 21 months (r = −0.68, P < 0.05), and 24 months (r = −0.61, P < 0.05) (Figures 1 and 2). Also, DAS28 values were related to NK cell count at 3 months (r = −0.25, P < 0.05), 6 months (r = −0.38, P < 0.05), 9 months (r = −0.37, P < 0.05), 12 months (r = −0.51, P < 0.01), 15 months (r = −0.59, P < 0.05), 18 months (r = −0.57, P < 0.05), 21 months (r = −0.61, P < 0.05), and 24 months (r = −0.58, P < 0.05). Subsequently, a linear regression least squares model backward method showed a significant correlation index between NK cell count modification at 3 months and SDAI/ DAS28 response at 6 months and 12 months (R2 = 0.73/0.71 and 0.70/0.71, respectively; P < 0.05), independent from other variables collected (CCP, rheumatoid factor, C-reactive protein, and erythrocyte sedimentation rate values, age, and disease duration) (Figures 1 and 2).
Figure 1.
Relationship between Simplified Disease Activity Index values measured (Y axis) and predicted (X axis) at 6 months with a linear regression model based on natural killer cell count at 3 months.
Abbreviation: SDAI, Simplified Disease Activity Index.
Figure 2.
Relationship between Simplified Disease Activity Index values measured (Y axis) and predicted (X axis) at 12 months with a linear regression model based on natural killer cell count at 3 months.
Abbreviation: SDAI, Simplified Disease Activity Index.
Discussion
The role of B cells in immunopathogenesis of RA has not been fully characterized, but several possible mechanisms of action have been proposed: B cells may function as an antigen presenting cells with costimulatory signals required for T cell CD4 regulation, they may also secrete proinflammatory cytokines (eg, tumor necrosis factor, interleukin 6, and other chemokines), and may regulate immune response during RA contributing to inflammation and bone erosions.21,22 There is also recent evidence regarding the involvement of NK cells in RA. NK cells originate from CD34+ hematopoietic progenitor cells and have been defined by flow cytometry as CD56+/CD16+ typical adult or mature cells, CD56+/CD16 immunoregulatory cells, and CD56−/CD16+ cytotoxic cells.23–25 NK cells have been detected in the synovium and elevated concentrations have been recorded in peripheral blood. NK cells may contribute to the pathogenesis of RA by perforin- or granzyme- mediated cytotoxicity and cytokine production.26–30 CD20-specific antibody rituximab has been proven to be a successful treatment for B cell malignancies and recently for RA. The binding of rituximab to CD20+ B cells results in B cell depletion through three mechanisms of action: antibody dependent complement mediated cytotoxicity, antibody-dependent cell-mediated cytotoxicity, and CD20+ B cell apoptosis. The depletion of peripheral B cells occurs immediately following the two infusions of rituximab, but different levels of clinical response have been achieved and therefore it is not possible to predict response on the basis of initial peripheral B cell depletion. NK cells appear to play a central role in mediating the effects of monoclonal antibody therapy including rituximab as effector cells of antibody-dependent cell-mediated cytotoxicity.20,31,32 In a preliminary study in 2009, a probable reverse relationship between DAS28 response and NK cell count in patients treated with a single course of rituximab was suggested.33
In the present long-term study, the relationship between NK cell activation (defined as an upregulation of CD54) mediated by rituximab and clinical response in patients with active RA was evaluated. Also, the use of NK cell count was tested as a predictor of clinical response. The chief finding was that the activation of NK cells 3 months after the first rituximab course was significantly related to clinical response at 6 months and 1 year, independent from other clinical variables such as C-reactive protein or CCP values, suggesting that NK activated cells can be used as an early predictor of clinical response.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cohen SB, Emery P, Greenwald MW, et al. REFLEX Trial Group. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 2.Stohl W, Looney RJ. B cell depletion therapy in systemic rheumatic diseases: different strokes for different folks? Clin Immunol. 2006;121(1):1–12. doi: 10.1016/j.clim.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Strand V, Kimberly R, Isaacs JD. Biologic therapies in rheumatology: lessons learned, future directions. Nat Rev Drug Discov. 2007;6(1):75–92. doi: 10.1038/nrd2196. [DOI] [PubMed] [Google Scholar]
- 4.Sfikakis PP, Boletis JN, Lionaki S, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52(2):501–513. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Yamamoto K, Takeuchi T, et al. A multicenter phase I/II trial of rituximab for refractory systemic lupus erythematosus. Mod Rheumatol. 2007;17(3):191–197. doi: 10.1007/s10165-007-0565-z. [DOI] [PubMed] [Google Scholar]
- 6.Kavanaugh A, Rosengren S, Lee SJ, et al. Assessment of rituximab’s immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann Rheum Dis. 2008;67(3):402–428. doi: 10.1136/ard.2007.074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi FD, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat Rev Immunol. 2006;6(10):751–760. doi: 10.1038/nri1935. [DOI] [PubMed] [Google Scholar]
- 8.Miyake S, Yamamura T. NKT cells and autoimmune diseases: unraveling the complexity. Curr Top Microbiol Immunol. 2007;314:251–267. doi: 10.1007/978-3-540-69511-0_10. [DOI] [PubMed] [Google Scholar]
- 9.Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer cell activity in families of patients with systemic lupus erythematosus: demonstration of a killing defect in patients. Clin Exp Immunol. 2005;141(1):165–173. doi: 10.1111/j.1365-2249.2005.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR. Natural killer T cells in families of patients with systemic lupus erythematosus: their possible role in regulation of IGG production. Arthritis Rheum. 2007;56(1):303–310. doi: 10.1002/art.22326. [DOI] [PubMed] [Google Scholar]
- 11.Shevach EM, Piccirillo CA, Thornton AM, McHugh RS. Control of T cell activation by CD4+CD25+ suppressor T cells. Novartis Found Symp. 2003;252:24–44. [PubMed] [Google Scholar]
- 12.Barath S, Aleksza M, Tarr T, Sipka S, Szegedi G, Kiss E. Measurement of natural (CD4+CD25high) and inducible (CD4+IL-10+) regulatory T cells in patients with systemic lupus erythematosus. Lupus. 2007;16(7):489–496. doi: 10.1177/0961203307080226. [DOI] [PubMed] [Google Scholar]
- 13.Breedveld F, Agarwal S, Yin M, et al. Rituximab pharmacokinetics in patients with rheumatoid arthritis: B-cell levels do not correlate with clinical response. J Clin Pharmacol. 2007;47(9):1119–1128. doi: 10.1177/0091270007305297. [DOI] [PubMed] [Google Scholar]
- 14.Dass S, Rawstron AC, Vital EM, Henshaw K, McGonagle D, Emery P. Highly sensitive B cell analysis predicts response to rituximab therapy in rheumatoid arthritis. Arthritis Rheum. 2008;58(10):2993–2999. doi: 10.1002/art.23902. [DOI] [PubMed] [Google Scholar]
- 15.Dass S, Burgoyne CH, Vital EM, et al. Reduction in synovial B cells after rituximab in RA predicts clinical response [abstract] Ann Rheum Dis. 2007;66(Suppl 2):ii, 90. [Google Scholar]
- 16.Teng YK, Levarht EW, Hashemi M, et al. Immunohistochemical analysis as a means to predict responsiveness to rituximab treatment. Arthritis Rheum. 2007;56(12):3909–3918. doi: 10.1002/art.22967. [DOI] [PubMed] [Google Scholar]
- 17.Thurlings RM, Vos K, Wijbrandts CA, Zwinderman AH, Gerlag DM, Tak PP. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis. 2008;67(7):917–925. doi: 10.1136/ard.2007.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42(2):244–257. doi: 10.1093/rheumatology/keg072. [DOI] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Bowles JA, Wang SY, Link BK, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108(8):2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Gamboa L, Brezinschek HP, Burmester GR, Dorner T. Immunopathologic role of B lymphocytes in rheumatoid arthritis: rationale of B cell-directed therapy. Autoimmun Rev. 2006;5(7):437–442. doi: 10.1016/j.autrev.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 23.Coppieters K, Dewint P, Van Beneden K, et al. NKT cells: manipulable managers of joint inflammation. Rheumatology (Oxford) 2007;46(4):565–571. doi: 10.1093/rheumatology/kel437. [DOI] [PubMed] [Google Scholar]
- 24.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003;100(16):9452–9457. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Namekawa T, Snyder MR, Yen JH, et al. Killer cell activating receptors function as costimulatory molecules on CD4+CD28null T cells clonally expanded in rheumatoid arthritis. J Immunol. 2000;165(2):1138–1145. doi: 10.4049/jimmunol.165.2.1138. [DOI] [PubMed] [Google Scholar]
- 26.Warrington KJ, Takemura S, Goronzy JJ, Weyand CM. CD4+, CD28− T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 2001;44(1):13–20. doi: 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25(1):47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 29.Mendes R, Bromelow KW, Westby M, et al. Flow cytometric visualization of cytokine production by CD3-CD56+ NK cells and CD3+CD56+ NK-T cells in whole blood. Cytometry. 2000;39(1):72–78. doi: 10.1002/(sici)1097-0320(20000101)39:1<72::aid-cyto10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 30.Reis EA, Athanazio DA, Lima I, et al. NK and NKT cell dynamics after rituximab therapy for systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int. 2009;29(4):469–475. doi: 10.1007/s00296-008-0719-0. [DOI] [PubMed] [Google Scholar]
- 31.Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(2):613–620. doi: 10.1002/art.21617. [DOI] [PubMed] [Google Scholar]
- 32.Assous N, Gossec L, Dieudé P, et al. Rituximab therapy in rheumatoid arthritis in daily practice. J Rheumatol. 2008;35(1):31–34. [PubMed] [Google Scholar]
- 33.Lurati A, Marrazza MG, Re KA, Scarpellini M. Relationship between NK cell activation and clinical response in rheumatoid arthritis treated with rituximab. Int J Biomed Sci. 2009;5(2):92–95. [PMC free article] [PubMed] [Google Scholar]