Abstract
Background
Soy isoflavone consumption may protect against breast cancer development. We conducted a Phase IIB trial of soy isoflavone supplementation, to examine its effect on breast epithelial proliferation and other biomarkers in the healthy high risk breast.
Methods
126 consented women underwent a random fine needle aspiration (rFNA); those with ≥ 4000 epithelial cells were randomized to a double-blind six-month intervention of mixed soy isoflavones (PTIG-2535) or placebo, followed by repeat rFNA. Cells were examined for Ki-67 labeling index (Ki-67 LI), and atypia. Expression of 28 genes related to proliferation, apoptosis and estrogenic effect was measured using quantitative RT-PCR. Hormone and protein levels were measured in nipple aspirate fluid (NAF). All statistical tests were 2-sided.
Results
98 women were evaluable for Ki-67 LI. In 49 treated women, the median Ki-67 LI was 1.18 at entry and 1.12 post-intervention, whereas in 49 placebo subjects it was 0.97 and 0.92 (p for between-group change 0.32). Menopausal stratification yielded similar results between groups, but within premenopausal soy-treated women, Ki-67 LI increased from 1.71 to 2.18 (p=0.04). We saw no treatment effect on cytologic atypia or NAF parameters. There were significant increases in the expression of 14/28 genes within the soy, but not the control group, without significant between-group differences. Plasma genistein values demonstrated excellent compliance.
Conclusions
A six-month intervention of mixed soy isoflavones in healthy, high risk adult western women did not reduce breast epithelial proliferation, suggesting a lack of efficacy for breast cancer prevention, and a possible adverse effect in premenopausal women.
Keywords: breast cancer, prevention, soy isoflavones
INTRODUCTION
The primary prevention of breast cancer currently rests on the selective estrogen receptor modulators (SERMs) tamoxifen (1) and, for postmenopausal women, raloxifene (2). However, toxicity concerns have rendered these generally unacceptable to healthy women (3–5). Dietary soy, or components of it such as genistein, may contribute to the lower breast cancer incidence seen in populations with high soy consumption, as demonstrated in several epidemiological investigations (6;7). Recent studies have also suggested a favorable effect on breast cancer survival (8). However, the beneficial effect of soy consumption on breast cancer risk may derive from exposure early in life, and the introduction of soy isoflavones into the diets of adult Western women may have minimal impact (9). Thus, well-designed prospective intervention studies are needed to support the epidemiological data, and allay concerns regarding a possible harmful pro-estrogenic effect of soy supplements, as suggested by several rodent studies (10;11). Since commercially available soy isoflavone supplements are being widely consumed by women of all age groups for a variety of reasons, it is important to know if soy isoflavones induce proliferation in the healthy breast. Furthermore, observation of an anti-proliferative effect would be grounds for wider investigation of soy isoflavones as breast cancer preventive agents in adult Western populations.
We undertook a Phase IIB placebo-controlled randomized trial of a mixed isoflavone compound in healthy, high risk women, to test the hypothesis that soy isoflavone supplementation for six months will decrease breast epithelial cell proliferation, measured as the Ki67 labeling index (Ki67 LI). This is the first report of a uniform high risk population undergoing a well-defined soy-isoflavone intervention with breast tissue biomarker analyses prior to and following the intervention.
METHODS
Study design
The study population consisted of healthy, non-pregnant and non-lactating women at increased risk for breast cancer, or women with a history of unilateral minimal risk breast cancer (Tis, or T1a-b, N0 breast cancer, when only the unaffected breast was studied). Subjects were recruited from the Lynn Sage Breast Center and the Bluhm Family Program for Breast Cancer Early Detection and Prevention of Northwestern Memorial Hospital. The study protocol was approved by the Institutional Review Board of Northwestern University and all subjects signed a document of informed consent. Eligible women were 25 to 55 years in age, with a 5-year Gail or Claus model risk estimate ≥ 1.66% for women over 40 years in age, ≥ 1.0% for those aged 30–39, and ≥ 0.1% for women aged 20–29. Adequate bone marrow, liver, kidney and thyroid function was required. Participants were asked to avoid soy containing foods and supplements, hormonal contraceptives, and hormone therapy, and kept a six-month diary of ingested foods, herbs, supplements and medications.
Participants underwent a two-week wash-out period where they avoided all soy foods, followed by the baseline study visit where breast epithelium was sampled by random fine needle aspiration (rFNA); nipple aspiration fluid (NAF) and peripheral blood was also collected. NAF samples were pooled if obtained from both breasts. The timing of the rFNA was in mid-luteal phase, predicted by the date of the last period and the usual length of the cycle. This was confirmed by the date of the next menstrual period, and the serum progesterone concentrations. The rFNA was performed as described by Fabian et. al. (12); samples from both breasts were pooled. Subjects with an epithelial yield ≥ 4000 cells were randomized 1:1 in a double-blind fashion to either one capsule per day of mixed soy isoflavones, or placebo, for a period of six months, followed by repeat rFNA, NAF, and blood collection. Stratifications factors included menopausal status, and history of unilateral cancer. Participants were designated postmenopausal if plasma FSH>30 mIU/ml, estradiol <30 pg/ml and progesterone <1 pg/ml with nomenstrual period within 6 months. The study agent, PTIG-2535, contained genistein 150 mg, daidzein 74 mg, glycitein 11 mg. PTIG-2535 and matched placebo pills were supplied by the National Cancer Institute Division of Cancer Prevention. Women were declared non-compliant if they consumed less than 80% of the dose based on pill counts, or if they had a lapse of one week or more during the last month of intervention.
Study endpoints
The primary endpoint was breast epithelial cell proliferation. Secondary endpoints included cytomorphologic assessment of atypia, and spectral imaging analysis of atypical features in epithelial cells (13). The expression of a panel of 28 genes (selected based on estrogen or genistein responsiveness, or because of an association with atypia in the breast)was measured in rFNA samples using quantitative RT-PCR. The breast endocrine environment was measured in NAF samples: estradiol, cathepsin D, insulin-like growth factor-1 (IGF-1) and epidermal growth factor (EGF). Plasma samples were assayed for genistein, equol, estradiol, progesterone, sex-hormone binding globulin (SHBG) and follicle-stimulating hormone (FSH).
Laboratory methods
Cytology and Ki-67 assessment
rFNA samples were rinsed into cold Cytolyte on ice and centrifuged immediately; the cell pellet was resuspended in 1ml Cytolyte and split into aliquots for RNA extraction (4/5th) and cytology (1/5th). RNA aliquots were resuspended in 1ml of TRIzol and stored at −80°C. Cytology aliquots were pre-filtered through a 20 μm nylon net filter (Millipore cat# NY200470); ThinPrep slides were prepared for Papanicolaou staining and immunohistochemistry. Immunostaining for Ki-67 was performed with mouse monoclonal antibody Clone MIB-1 (DakoCorp., M7240), in batches containing pre-and post-intervention samples from each subject(14). Each run included a reference sample obtained by pooling of several rFNA aspirations of prophylactic mastectomy specimens, and a negative control. Assessment of Ki-67 staining was by manual touch counts of a minimum of 500 epithelial cells on digitized images, using Metamorph software; 10% of samples were blindly recounted by the same observer (DI), and 20% of samples were assessed by a different observer using image analysis which involved standardized automatic acquisition (TissueFAXS 1.2.4 software, TissueGnostics, Vienna, Austria) and a motor stage (Märzhäuser, Wetzlar, Germany). The intra-observer correlation was 0.88 and the inter-observer correlation was 0.86. The mean Ki-67 LI for the positive control slide was 4.27 (range 3.99–5.10, standard deviation 0.42). Cytologic atypia evaluation was performed on Papanicolau stained Thin Prep slides using standard criteria (15;16), which were also used for spectral spatial imaging. Cell clusters were used to generate image stacks with the Nuance LCTF-based imaging system (CRI Inc). To build the algorithmic model, image stacks were analyzed using a neural network-based artificial intelligence system now distributed commercially as the InForm system. Manual painting of atypical (red) and benign (green) features was followed by application of adiagnostic algorithmic previously developed and tested in benign versus malignant breast cytological samples(17); the image data was collected as percent pixels assigned as “atypical”.
RNA analyses
Total mRNA was extracted from rFNA samples using TRIzol (Sigma-Aldrich, USA) and purified using the RNeasy Plus Micro Kit (Qiagen # 74034). A total of 100 ng of RNA was reverse transcribed using the High Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Austin, TX). Nine participants whose clinical samples did not yield 100 ng RNA were not analyzed. Amplicons of interest were linearly amplified using the TaqMan® PreAmp Master Mix kit (Applied Biosystems, Austin, TX) with 10 cycles of amplification. We selected 28 genes including 14 genes reported as the molecular targets of genistein in vitro (18;19), 9 ERα related genes identified in benign breast samples (our unpublished data) and 5 genes associated with breast epithelial atypia (20). Two housekeeping genes (GAPDH and HPRT1) were chosen for normalization. Taqman low density gene expression assays (TLDA) were preloaded in 384 well micro fluidic cards (each gene in triplicate) from Applied Biosystems. Assays were designed with small amplicons (<100 bp) to enhance detection sensitivity. Real Time PCR reactions were carried out in an Applied Biosystems 7900HT machine. For each gene of interest, expression levels were normalized to the average expression of GAPDH and HPRT1. Seven samples with sufficient cDNA to allow qRT-PCR without amplification were checked against the results from the post-amplification TLDA assays to confirm linear amplification; the values for amplified and unamplified cDNA were highly correlated (R2 = 0.95); the plot resulting from this comparison is shown in Supplemental Figure 1.
Plasma Hormone Assays
Plasma was assayed for estradiol, progesterone, FSH and SHBG. Radioimmunoassay (RIA) kits were purchased from Diagnostic Systems Laboratories (DSL) for estradiol and progesterone quantification. An enzyme immunoassay (EIA) purchased from Alpco Diagnostics was used for FSH quantification. An Iso-Data 20/20 Series gamma counter was used for measurements in the RIAs, and a BIO-TEK Synergy HT plate reader was used for the measurements in the EIAs.
Plasma genistein and equol assays
High pressure liquid chromatography (HPLC) analysis with electrochemical detection of plasma soy isoflavones was carried out using the procedure of Gamache & Acworth with slight modifications (21;22). Genistein and equol concentrations were measured taking into account the recovery of an estriol-glucuronide internal control at a concentration of 2 nmol/ml (973 ng/ml) (23). Estriol recovery was 75% (CV 15.5%) in this series of 190 plasma samples, excluding 5 outliers. Estriol serum levels in pre-and post-menopausal women are in the range of 6–12 pg/ml, therefore endogenous estriol was not a concern. The CVs for the positive genistein, equol, and estriol spiked methanol controls were 18, 9, and 7%, respectively. The sensitivity of this assay for genistein and equol is approximately 3 ng/ml.
NAF hormone and protein assays
NAF volume was measured in calibrated capillary tubes and diluted in phosphate buffered saline. Estrogens were extracted into ethyl acetate-hexane (3:2), and the extract was fractionated by HPLC on a C18 column as described previously (24) The recovery of E2 averaged 78.3%, with a lower limit of detection of6.25 pg/ml. The intra-and interassay %CVs were: E2, 4.89 and 6.55; E1 5.38 and 6.82; EGF, 5.38 and 20.2; Cathepsin D, 8.63 and 36.6; and IGF-I, 6.68 (BCF does not have detectable IGF-1 for calculation of interassay variation). Modified RIA kits from DSL were used for estradiol quantification (25). Cathepsin D and epidermal growth factor (EGF) were measured in the aqueous fraction with EIA kits from Calbiochem and Alpco Diagnostics respectively. Insulin-like growth factor 1 (IGF-1) was measured in the aqueous fraction with a RIA from Alpco Diagnostics.
NAF isoflavones
150 μL diluted NAF was mixed with 15 μL triply labeled 13C–standards of daidzein, genistein, and equol (purchased from the University of St. Andrews, Scotland), incubated with beta-glucuronidase and arylsulfatase. This mixture was extracted with methyl tertiary-butyl ether and analyzed by LCMS using a Gemini C18analytical column (150 × 2.0; 5μm; Phenomenex, Torrance, CA) with the following linear gradient of A= methanol/acetonitrile (1:1) and B= water at 0.2 mL/minute (%B): 40% to 60% in 2.5 minutes, hold at 60% for 5.5 minutes and equilibrate at 40% for 2 minutes before subsequent injections. Electrospray ionization followed by high-accuracy orbitrap mass spectrometry (model Exactive, ThermoFisher, Waltham, MA) in negative mode was applied for all analytes according to the published method (26). The lower limits of detection for daidzein, genistein, and equol were 0.2, 0.5, and 1.2 ng/mL aq. fraction. The intra-and inter-assay % CVs were 9–13% for all analytes in a concentration range of 5–30 ng/mL.
Statistical methods
We planned to accrue 150 women and randomize 120, expecting that 80% of subjects would yield sufficient epithelial cells for analysis (≥ 4000 cells). With a 28% drop out rate (including women who had insufficient cells for analysis the 6-month time-point), we planned a total of 90 women (45 per group) for final analysis. We estimated a median post-intervention decrease in the primary endpoint (Ki-67 LI of epithelial cells) of 1.5%in the soy group, compared to a median change of zero in the control group. Assuming a standard deviation of 1.5–2%, this would provide over 90% power with 45 subjects per group. Interim analyses were planned to identify evidence for a systemic estrogenic effect of the soy-isoflavone supplement, defined as an increase in the one-month plasma in SHBG of 1.5 times the baseline level.
The baseline demographic characteristics between treatment and control groups were compared using the Wilcoxon rank-sums test for continuous variables and Fisher’s exact test for categorical variables. Analyses of cellular parameters were adjusted for cell number. The effects of treatment were assessed within groups (month 6 minus baseline) using the signed-rank test and between groups (treated difference minus control difference) using the Wilcoxon rank-sums test. Women with plasma equol concentrations >5 ng/ml were designated asequol producers (27). For NAF data, since there was a substantial proportion of non-detectable values, we first calculated Month 6 minus baseline changes and then categorized these changes into tertiles. We then compared frequencies of subjects in each tertile between groups using Fisher’s exact test. For gene expression data, we obtained cycle threshold (Ct) values from the PCR experiments; Ct outliers within triplicates (determined using the Grubbs (1950) method) were omitted. Ct values were averaged across triplicates by subject, gene, and visit. Genes were normalized by subtracting the mean of the housekeeping genes GAPDH and HPRT1 for each subject, gene, and visit (ΔCt). The normalized baseline, month-6, and month-6 minus baseline values (ΔΔCt) were exponentiated by a negative power of 2. The means, standard deviations, and 95% confidence intervals were calculated for the exponentiated data. The month-6 minus baseline differences between groups were tested using the unpaired t-test, while differences within groups were tested using the paired t-test. We adjusted p-values from these tests via the Benjamini-Hochberg (1995) approach. We also conducted a global analysis in order to examine if there was an overall difference among treatment and menopausal groups in Month 6 minus baseline changes across all 30 genes combined. Global tests here were based on an analysis of variance (ANOVA). In order to examine the similarity among the samples on gene expression profiles, a clustering analysis was performed using Cluster v2.11 and TreeView v1.6 from Michael Eisen. All statistical tests were two-sided.
Results
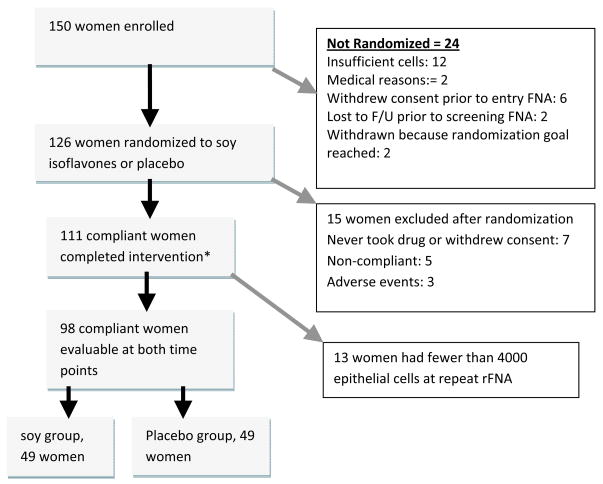
Of 150 women consented, 138 underwent the entry rFNA procedure, with 12 (8.7%) yielding insufficient cells, so that 126 subjects were randomized. Of these, 98 (77.8%) had >4000 epithelial cells in rFNA samples pre-and post-intervention, met the criteria for compliance, and were evaluable for the primary endpoint of Ki-67 labeling of epithelial cells at both time points. The CONSORT diagram is shown in Figure 1, and Table 1 shows the evaluable participant characteristics. Mid-luteal phase timing of the rFNA was achieved at both time points in 43/53 (81%) premenopausal women. In 10 women, luteal phase timing could not be confirmed because the cycles had become irregular. Since results were similar in analyses restricted to the 43 women who were in luteal phase at both time points and in all 53 premenopausal women, we have presented results for all premenopausal women. Compliance to the study regimen was excellent among the 98 women included in the final analysis, as shown in Table 2. The median plasma genistein levels were 156 ng/ml in postmenopausal women and 205 ng/ml in premenopausal women in the treated group, compared to zero in the control group. The median plasma concentration of FSH and SHBG, and ratio of estradiol to SHBG, did not change following intervention, in both pre-and postmenopausal women (see Table 2).
Figure 1.
CONSORT diagram: retention of participants through the study.
* One woman underwent post-intervention rFNA after three months because of heavy bleeding from pre-existing uterine fibroids but was included since all study parameters were complete and evaluable.
Table 1.
Characteristics of Evaluable Participants.
| Soy group (N = 49) | Control group (N = 49) | p-value | |
|---|---|---|---|
| Age in years (I-Q range) | 48.00 (42.00, 53.00) | 50.00 (46.00, 55.00) | 0.27 |
| Race | |||
| African American | 4 (8.2%) | 6 (12.2%) | 0.74 |
| White | 45 (91.8%) | 43 (87.8%) | |
| Ethnicity | |||
| Gail risk-estimate (5-years) | 2.10 (1.75, 3.15) | 2.20 (1.80, 2.70) | 0.69 |
| Gail risk estimate (lifetime) | 19.10 (15.20, 26.50) | 17.30 (15.00, 25.80) | 0.45 |
| Menopause Status at study entry | |||
| Pre | 28 (57.1)% | 25 (51.0%) | 0.69 |
| Post | 21 (42.9%) | 24 (49.0%) | |
| Menstrual Phase at rFNA | |||
| Follicular at both | 2 (7.1%) | 4 (16.0%) | 0.28 |
| Luteal at both | 25 (89.3%) | 18 (72.0%) | |
| discordant | 1 (3.6%) | 3 (12.0%) | |
| Soy Stratification | |||
| Premenopausal, no cancer | 23 (52.3%) | 21 (47.7%) | 0.85 |
| Postmenopausal, no cancer | 14 (48.3%) | 15 (51.7%) | |
| History of ER-cancer* | 6 (60.0%) | 4 (40.0%) | |
| History of ER+ cancer* | 9 (60.0%) | 6 (40.0%) |
History of unilateral breast cancer with all systemic therapy completed at least one year previously, only unaffected breast sampled.
Table 2.
Plasma genistein and endocrine parameters in treatment and placebo groups.
| Parameter | Soy group (n=49) | Placebo group (n=49) | |||||
|---|---|---|---|---|---|---|---|
| N | Entry | Six months | Entry | Six months | Between-group difference | P value | |
| Plasma genistein in units ng/ml (Median and inter-quartile range) | |||||||
| All patients | 97 | 0 (0, 0) | 174 (113, 377) | 0 (0, 0) | 0 (0, 0) | 174 (113, 377) | <0.0001 |
| Post-menopausal | 44 | 0 (0, 0) | 156 (21, 391) | 0 (0, 0) | 0 (0, 0) | 156 (21, 391) | <0.0001 |
| Pre-menopausal | 53 | 0 (0, 0) | 205 (124, 374) | 0 (0, 0) | 0 (0, 0) | 205 (124, 374) | <0.0001 |
| Median Plasma estradiol pg/ml (Median and inter-quartile range) | |||||||
| All patients | 97 | 26.12 (12.73, 54.59) | 26.01 (15.68, 59.16) | 17.01 (10.18, 38.52) | 21.74 (13.46, 55.89) | 4.12 (−6.52, 17.91) | 0.77 |
| Post-menopausal | 44 | 11.54 (6.61, 16.62) | 16.46 (12.70,20.59) | 10.31 (9.33, 16.62) | 14.04 (12.00, 19.20) | 2.90 (−1.54, 9.01) | 0.36 |
| Pre-menopausal | 53 | 45.29 (28.71, 72.35) | 47.82 (26.63, 72.93) | 36.32 (20.38, 70.43) | 51.94 (43.38, 72.92) | 8.94(−19.86, 33.42) | 0.57 |
| Median sex hormone-binding globulin nmol/L (Median and inter-quartile range) | |||||||
| All patients | 97 | 61.25 (45.52,104.35) | 71.30 (47.22,97.34) | 85.84 (55.26,118.71) | 78.47(55.84,111.85) | −5.34 (−18.82, 15.11) | 0.43 |
| Post-menopausal | 44 | 78.01(54.98,100.97) | 72.94(56.59,119.38) | 103.37(58.27,122.09) | 86.05(57.19,121.63) | −4.98(−17.82, 15.28) | 0.57 |
| Pre-menopausal | 53 | 55.13 (39.56,105.54) | 63.21 (42.85,91.02) | 77.28(51.79,118.71) | 74.65(53.68,111.85) | −5.34 (−21.44, 9.45) | 0.56 |
| Median Ratio of estradiol to SHBG (Median and inter-quartile range) | |||||||
| All patients | 97 | 0.32 (0.18, 0.83) | 0.37(0.20, 0.87) | 0.24 (0.12, 0.51) | 0.40 (0.12, 0.80) | 0.08 (−0.05, 0.26) | 0.48 |
| Post-menopausal | 44 | 0.16 (0.10, 0.21) | 0.20 (0.13, 0.34) | 0.14 (0.08, 0.19) | 0.18 (0.09, 0.36) | 0.03 (−0.02, 0.11) | 0.72 |
| Pre-menopausal | 53 | 0.73 (0.37, 1.31) | 0.67 (0.37, 1.15) | 0.46 (0.30, 0.77) | 0.80 (0.50, 1.10) | 0.15 (−0.23, 0.58) | 0.28 |
| Median Follicle stimulating hormone mIU/ml (Median and inter-quartile range) | |||||||
| All patients | 97 | 10.90 (5.53, 69.76) | 17.43 (5.17, 84.79) | 34.78 (4.24, 72.62) | 49.67 (5.80, 77.77) | 2.75 (−4.15, 11.66) | 0.45 |
| Post-menopausal | 44 | 71.72 (48.73, 90.67) | 85.63 (76.52, 93.54) | 70.85 (50.23, 96.13) | 77.40 (65.82, 95.68) | 6.75 (−4.09,26.87) | 0.72 |
| Pre-menopausal | 53 | 5.73 (2.46, 8.30) | 6.01 (0.01, 9.64) | 4.24 (1.50, 12.92) | 5.80 (0.01, 20.97) | 0.00 (−4.15, 5.87) | 0.80 |
The results related to proliferation and cytological features of the epithelial cells are shown in Table 3. The mean epithelial cell yield at baseline was 40,030 and was 47,867 post-intervention. As expected, the baseline Ki-67 LI was significantly higher in premenopausal than in postmenopausal women (1.79 vs. 0.76, p<0.001), and was higher in samples obtained in luteal phase than in follicular phase (1.95 vs. 1.26, p<0.04). In the control group, the Ki-67 LI was concordant between entry and 6-month samples, with a Pearson R2=0.61 (p<0.0001). The change in Ki-67 LI (i.e. month-6 minus baseline values) was similar between the soy and placebo groups in the entire study population. In contrast, following menopausal stratification, we observed a statistically significant increase in Ki-67 LI from baseline to post-intervention within the premenopausal soy-treated women (1.71 versus 2.18, p=0.04), but not in control premenopausal women (1.90 versus 1.94, p=0.56). We then compared the median change in Ki-67 LI between treated and control premenopausal subjects and found no significant difference (0.19%, IQ range -0.46, 1.07, p=0.31). Among postmenopausal women, there were no significant differences in Ki-67 labeling, within or between treated and placebo groups, comparing baseline to post-intervention values. Notably, the direction of the post-intervention change in Ki-67 LI was significantly different between pre and postmenopausal women (+0.19 versus -0.13, p=0.03). We did find a significant positive relationship between Ki-67 LI and cytologic atypia (p<0.02) and with the life-time Gail risk estimate (p=0.005), despite a significant negative association with age (p=0.001). However, the association between Ki-67 LI and epithelial cell number was weak and non-significant.
Table 3.
Cellular parameters in treatment and placebo groups.
| Parameter | Soy group (n=49) | Placebo group (n=49) | Between group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Six months | p | Entry | Six months | p | Difference | P value | ||
| Median Ki-67 Labeling Index (inter-quartile range) | |||||||||
| All subjects | 98 | 1.17 (0.66, 1.93) | 1.09 (0.75, 2.33) | 0.82 | 0.97 (0.70, 1.90) | 0.92 (0.59, 1.96) | 0.14 | −0.03(−0.42, 0.08) | 0.24 |
| Post-menopausal | 45 | 0.63 (0.52, 1.08) | 0.79 (0.35, 1.00) | 0.56 | 0.79 (0.55, 1.05) | 0.63(0.40, 0.92) | 0.22 | −0.13(−0.37, 0.20) | 0.73 |
| Pre-menopausal | 53 | 1.71 (1.12, 2.35) | 2.18 (1.18, 3.04) | 0.04 | 1.90 (0.88, 2.33) | 1.94 (0.92, 2.55) | 0.56 | 0.19(−0.46, 1.07) | 0.31 |
| Proportion of women with Atypical Cytology | |||||||||
| All subjects | 98 | 42.9% | 53.1% | 0.79 | 40.8% | 53.1% | 0.31 | −2.1% | 0.83 |
| Post- menopausal | 45 | 33.3% | 23.8% | 0.68 | 33.3% | 33.3% | 0.99 | −9.5% | 0.72 |
| Pre- menopausal | 53 | 50.0% | 75.0% | 0.29 | 48.0% | 72.0% | 0.15 | 1.0% | 0.99 |
| Median Masood Score (inter-quartile range) | |||||||||
| All subjects | 98 | 14.0 (13.0, 15.0) | 14.0 (12.0, 15.0) | 0.68 | 13.0 (13.0, 15.0) | 14.0 (13.0, 15.0) | 0.37 | 0.0 (−1.0, 1.0) | 0.64 |
| Post- menopausal | 45 | 14.0 (13.0, 15.0) | 13.0 (12.0, 14.0) | 0.04 | 13.0 (13.0, 14.5) | 13.0 (12.0, 15.0) | 0.32 | 0.0 (−1.0, 0.0) | 0.15 |
| Pre- menopausal | 53 | 14.0 (13.0, 15.5) | 15.0 (14.0, 16.0) | 0.33 | 14.0 (13.0, 16.0) | 15.0 (14.0, 16.0) | 0.13 | 1.0 (−1.0, 2.0) | 0.62 |
| Atypical features on spectral Imaging (inter-quartile range) | |||||||||
| All subjects | 94 | 0.42 (0.15, 0.60) | 0.32(0.10, 0.58) | 0.50 | 0.42 (0.15, 0.60) | 0.51(0.19, 0.72) | 0.63 | 0.02(−0.28, 0.23) | 0.47 |
| Post- menopausal | 43 | 0.41 (0.10, 0.51) | 0.38 (0.08, 0.56) | 0.57 | 0.40 (0.12, 0.56) | 0.29 (0.14, 0.58) | 0.70 | 0.04 (−0.21, 0.24) | 0.81 |
| Pre- menopausal | 51 | 0.47 (0.23, 0.66) | 0.28(0.10, 0.60) | 0.29 | 0.45 (0.18, 0.75) | 0.62 (0.28, 0.77) | 0.66 | 0.00(−0.30, 0.21) | 0.32 |
There were no significant between-group changes in morphologic features of the epithelial cells, measured categorically as the presence or absence of cytologic atypia, or as assessed using the Masood score. This was true for all women, and for tests stratified by menopausal status (Table 3). Despite a borderline improvement in the post-intervention Masood score within the soy-treated postmenopausal group (from 14 to 13, p=0.04), there was no significant difference between treated and control postmenopausal women. Similarly, the presence of atypical features by spectral-spatial imaging showed that the median fraction of epithelial clusters showing atypical features was similar pre and post intervention, within and between groups.
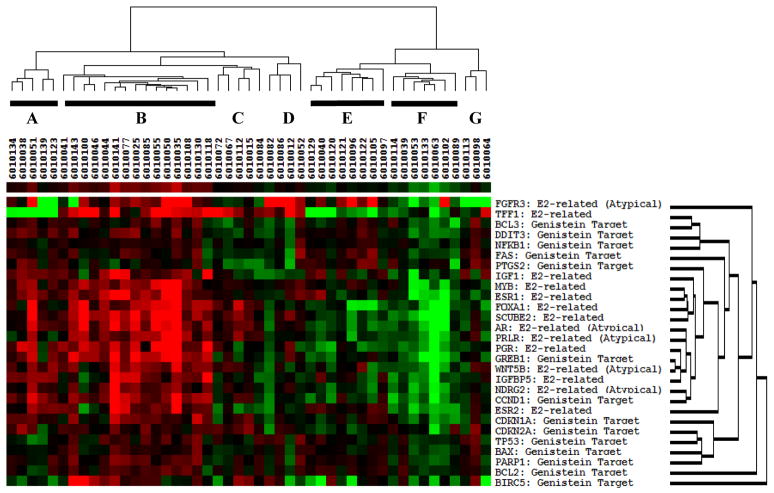
Gene expression patterns for the 28 genes evaluated were similar at baseline between treated and control women; with GAPDH and HPRT1 as reference genes, the mean fold-expression of the genes of interest was 1.56 for controls and 1.42 for the soy group (p=0.28), with no significant effect of menopausal status (p=0.11). Within the soy group, we observed a significant increase in expression of 14/28 genes from baseline to post-intervention, with adjusted p values ranging from 0.017 for BCL-2, to 0.052 for FAS (Table 4). These included 7 that had been selected on the basis of genistein response (BCL-2, CDKN1A, CDKN2A, DDIT3, FAS, PARP-1, and TP53), five that were chosen based on estrogen response (ESR1, FOXA1, MYB, PGR, SCUBE2) and two that were selected because of an association with breast epithelial atypia (AR and Wnt5B). In contrast, there were no significant changes in the expression of any of the 28 genes within the control group. The mean fold-change from baseline to 6-months across all 28 genes in the soy-treated women was 1.56, versus 1.25 for control subjects (p=0.02). At the 6-month time-point, the mean fold-expression (relative to housekeeping genes) in the soy group was 1.66, versus 1.31 for the control subjects (p=0.0001). On examining month-6 minus baseline differences in individual gene expression between soy and placebo groups, four genes showed a significantly larger increase in the soy than in the placebo group (ESR1, FAS, FOXA1, MYB) but these increases were not significant following Benjamini-Hochberg adjustment. Detailed results for individual genes within and between groups are shown in Table 4. A heat map of gene expression patterns of month-6 minus baseline differences in the soy group is shown in Figure 3. Samples clustered into two branches, with overall expression increasing in the first branch (cluster A, B, C and D) and decreasing in the second branch (cluster E, F and G). In cluster A and B (20 subjects), the expression of estrogen-responsive and epithelial atypia-associated genes was dramatically stimulated, while the expression of genistein target genes was moderately increased. On the contrary, in cluster E and F (15 subjects) the expression of estrogen-responsive genes and epithelial atypia-associated genes was dramatically suppressed, and the expression of genistein target genes was moderately decreased. The samples in cluster C, D and G (12 subjects) showed intermediate expression pattern between cluster A/B and cluster E/F.
Table 4.
Changes in expression of individual genes, month-6 minus baseline values
| Differences within the soy group | Differences within the control group | P value for between-group differences | ||||||
|---|---|---|---|---|---|---|---|---|
| Gene Name | Mean fold-change | SD | Adjusted p | Mean fold-change | SD | Adjusted p | Raw p | Adjusted p |
| Genistein molecular targets | ||||||||
| BAX-Hs00180269_m1 | 1.15 | 0.56 | 0.123 | 0.98 | 0.62 | 0.874 | 0.178 | 0.654 |
| BCL2-Hs99999018_m1 | 1.34 | 0.58 | 0.017 | 1.24 | 0.82 | 0.109 | 0.499 | 0.705 |
| BCL3-Hs00180403_m1 | 1.21 | 0.79 | 0.123 | 1.11 | 0.99 | 0.512 | 0.585 | 0.705 |
| BIRC5-Hs00153353_m1 | 1.79 | 2.8 | 0.109 | 1.91 | 3.62 | 0.15 | 0.887 | 0.887 |
| CCND1-Hs00765553_m1 | 3.27 | 8.65 | 0.123 | 2.21 | 3.85 | 0.101 | 0.455 | 0.705 |
| CDKN1A-Hs00355782_m1 | 1.58 | 1.4 | 0.035 | 1.24 | 1.28 | 0.27 | 0.226 | 0.654 |
| CDKN2A-Hs99999189_m1 | 1.43 | 0.9 | 0.025 | 1.26 | 0.82 | 0.101 | 0.349 | 0.654 |
| DDIT3-Hs01090850_m1 | 1.37 | 0.92 | 0.035 | 1.17 | 1.11 | 0.353 | 0.342 | 0.654 |
| FAS-Hs00163653_m1 | 1.32 | 0.84 | 0.052 | 1.01 | 0.62 | 0.954 | 0.050 | 0.447 |
| GREB1-Hs00536409_m1 | 4.39 | 11.3 | 0.106 | 2.98 | 6.74 | 0.109 | 0.469 | 0.705 |
| NFKB1-Hs00765730_m1 | 1.14 | 0.43 | 0.089 | 1.05 | 0.48 | 0.549 | 0.315 | 0.654 |
| PARP1-Hs00911369_g1 | 1.42 | 0.79 | 0.022 | 1.22 | 1.02 | 0.189 | 0.302 | 0.654 |
| PTGS2-Hs00153133_m1 | 1.24 | 0.8 | 0.109 | 1.29 | 1.22 | 0.159 | 0.791 | 0.844 |
| TP53-Hs01034253_m1 | 1.29 | 0.71 | 0.035 | 1.21 | 0.73 | 0.109 | 0.595 | 0.705 |
| Estrogen responsive genes | ||||||||
| ESR1-Hs00174860_m1 | 2.96 | 4.33 | 0.027 | 1.46 | 1.82 | 0.15 | 0.034 | 0.447 |
| ESR2-Hs00230957_m1 | 2.49 | 5.25 | 0.109 | 1.59 | 2.51 | 0.166 | 0.298 | 0.654 |
| FOXA1-Hs00270129_m1 | 2.9 | 4.64 | 0.035 | 1.47 | 1.76 | 0.123 | 0.056 | 0.447 |
| IGF1-Hs01547657_m1 | 2.83 | 6.74 | 0.122 | 1.88 | 2.23 | 0.046 | 0.369 | 0.654 |
| IGFBP5-Hs01052296_m1 | 3.2 | 9.49 | 0.163 | 1.63 | 1.94 | 0.1 | 0.282 | 0.654 |
| MYB-Hs00920568_m1 | 2.44 | 3.15 | 0.027 | 1.3 | 1.21 | 0.152 | 0.025 | 0.447 |
| PGR-Hs01556702_m1 | 3.91 | 7.58 | 0.046 | 12.61 | 68.36 | 0.303 | 0.388 | 0.654 |
| SCUBE2-Hs00221277_m1 | 4.03 | 7.16 | 0.035 | 2.51 | 6.1 | 0.15 | 0.278 | 0.654 |
| TFF1-Hs00907239_m1 | 14.31 | 42.9 | 0.101 | 27.87 | 139.5 | 0.25 | 0.526 | 0.705 |
| Breast epithelial atypia associated genes | ||||||||
| PRLR-Hs00168739_m1 | 2.38 | 4.01 | 0.082 | 1.6 | 1.89 | 0.101 | 0.244 | 0.654 |
| AR-Hs00171172_m1 | 3.06 | 4.56 | 0.027 | 1.76 | 3.31 | 0.171 | 0.122 | 0.654 |
| FGFR3-Hs00997397_m1 | 18.3 | 52.5 | 0.089 | 6.33 | 15.85 | 0.089 | 0.146 | 0.654 |
| NDRG2-Hs00212263_m1 | 1.53 | 1.8 | 0.109 | 1.33 | 1.61 | 0.216 | 0.579 | 0.705 |
| WNT5B-Hs00364142_m1 | 1.92 | 1.92 | 0.025 | 2.35 | 4.54 | 0.109 | 0.555 | 0.705 |
| Housekeeping genes | ||||||||
| GAPDH-Hs00266705_g1 | 1.01 | 0.16 | 0.697 | 1.02 | 0.16 | 0.354 | 0.68 | 0.751 |
| HPRT1-Hs01003267_m1 | 1.01 | 0.15 | 0.601 | 1 | 0.15 | 0.954 | 0.653 | 0.746 |
NAF collection was attempted on all women, and was successful in 46 women at both time-points (26 in the soy group, and 20 in the control group). The tertile distribution of post-intervention change in NAF volume, estradiol, and protein concentrations (total protein, EGF, cathepsin-D, IGF-1) was compared between groups. Numerical values for each parameter are shown in Supplemental Table 1. We found no significant differences in the tertiles of changes in any of these parameters between soy and placebo groups. However, the median NAF genistein concentration was significantly different, being 64.2 ng/ml in the treated women, and 6.2 ng/ml in the placebo group (p<0.0001). Within the soy treated cohort, there was no correlation between NAF and plasma genistein concentrations at the 6-month time point (p=0.54), or between NAF genistein or daidzein values and Ki-67 LI in breast epithelial cells at six months (R2=0.02 and p=0.32 for genistein, R2=.01 and p=0.56 for daidzein).
Plasma equol concentrations at entry were equivalent in the soy and placebo groups (2.7 ng/ml in both groups) but were markedly different following intervention, with the treated women displaying a mean plasma equol concentration of 673 ng/ml, compared to 5.6 ng/ml in the placebo group. Measurable plasma equol was >5 ng/ml 30/48 women (62.5%) who were designated equol-producers. When compared to control women, the baseline to 6-month change in equol producers showed no significant differences in Ki67 LI, cytologic atypia, median percent atypical features, or estradiol values (Supplemental Table 2), although within premenopausal equol-producers the median change in Ki-67 LI was +0.77 compared to −.02 in controls (p=0.44).
Serious adverse events occurred in seven women (5 in the soy group) while on study, all considered unrelated to study drug. These included two events related to uterine fibroids (anemia requiring hospitalization in one woman and surgery for symptoms in a second); grade 3 depression in a patient with a history of bipolar disorder 47 days after the last dose; grade 3 back pain hospitalized for surgery 24 days after initiation of the study drug; dyspnea 57 days after drug initiation. One placebo subject developed breast cancer; one soy-treated woman discontinued participation because her TSH levels rose. Additional data regarding the distribution of adverse events is presented in Supplemental Table 3.
Discussion
The soy isoflavones are prime dietary candidates for breast cancer prevention, with a wealth of supporting epidemiological and laboratory data; recent studies provide additional support for a protection against breast cancer causation, and relapse (6–8). However, these favorable effects of soy consumption are found mainly in Asian populations (7), with the possible explanation that intake early in life is key(9). Soy consumption by Western women at risk for breast cancer, and breast cancer survivors, has been deterred by rodent data showing a cancer-promoting effect of soy components (10; 11). Unbiased data on breast outcomes following introduction of soy components into the diets of adult Western women are lacking. We conducted a randomized Phase II trial using unconjugated mixed soy isoflavones in a dose that approximates that of the upper quartile of soy-consuming Far-East Asians (8; 28). We used a two-week wash-out period prior to randomization, and provided all participants with a list of soy-containing foods to avoid during the study period. The median post-intervention plasma genistein concentrations (174 ng/ml in treated and 0 ng/ml in placebo women) show that our participants adhered well to the study regimen.
We found no significant favorable effect on the primary endpoint of epithelial cell proliferation, as measured by Ki67 labeling. However, among treated premenopausal women, there was a relative increase of 27% in the post-intervention Ki67 LI following soy supplementation which was statistically significant (from 1.71 to 2.18, p=0.04). In contrast, the premenopausal placebo group, the relative post-intervention increase in Ki-67 LI was a non-significant 2%. However, the post-intervention change in Ki67 LI between treated and placebo premenopausal women was not significantly different (relative change 27% vs. 2%, p=0.31). Among postmenopausal women, all Ki67 comparisons were entirely null. Of interest, the effect of soyon Ki67 LI differed by menopausal status, with a median decrease of 0.13%in the postmenopausal treated group, whereas in premenopausal women it increased by 0.19% (p=0.03).
The reason for the apparent stimulatory effect upon breast epithelial cell proliferation in premenopausal women is not clear, but a pro-estrogenic effect of soy is suspect, possibly including an interaction with progesterone, since the great majority of our premenopausal samples were obtained in luteal phase. In premenopausal treated women, median NAF estradiol content was 116.4 at baseline and 206.3 post-intervention; this was not statistically significant, but may help to explain the higher Ki-67 LI in premenopausal women. A previous study of 2–4 weeks of soy isoflavone supplementation in premenopausal women showed no effect on Ki-67 labeling of benign breast tissue, but the tissue assessment was performed only post-intervention (29). Our results for postmenopausal women are in agreement with studies of soy supplementation in oophorectomized macaque monkeys, which failed to show an effect on mammary gland proliferation (30;31).
We used careful quality-control for the measurement of the primary endpoint (Ki67 LI); we counted a mean of 1,593 cells; the intra-observer and inter-observer correlations were high (0.88 and 0.86 respectively) and a positive control sample from pooled ex-vivo aspirations of prophylactic mastectomy specimens was included in each batch, with excellent batch-to-batch concordance of Ki-67 LI. We did not restrict entry to women with atypical cytology, a strategy that has been proposed to ensure that women entering Phase II prevention trials have high starting Ki-67. However, the power of finding a difference between groups is driven by the size of the difference and the variation of the data; we observed a similar IQ range in premenopausal women (baseline median Ki-67 LI 1.85, I-Q range 0.99, 2.33) and in postmenopausal women (baseline median Ki-67 LI 0.79, I-Q range 0.55, 1.08), and our I-Q ranges are smaller than other studies (32). Thus it is unlikely that a higher starting Ki-67 LI would have changed the outcome of our study; but it would have rendered accrual more challenging. Of note, the Masood score at entry in our study population was 13 or greater, the higher end of the hyperplasia without atypia range (11–14).
Among pre-menopausal and postmenopausal women, we did not observe any significant changes in cytologic atypia, Masood score, or spectral imaging.
The study plan included an analysis of expression of a panel of 28 genes, pre and post intervention, selected based on published expression profiles in genistein-treated breast cancer cells, genes involved in estrogen response, and those associated with the presence of epithelial atypia. We saw no significant change in individual gene expression from baseline to post-intervention in the placebo group. However, the treated group did demonstrate a soy isoflavone signal, with a significant increase in the expression of 14/28 genes as well as a significantly higher global expression at six months compared to control subjects (p=0.0001).
The specific genes displaying increased expression within the soy group showed a mixed pattern, with more adverse than beneficial effects. For example, ESR1 expression was increased, suggesting and anti-estrogenic effect, but FOXA1, MYB, PGR, TIFF1 and SCUBE-2 were also increased, suggesting estrogenicity. On the cluster trees among the genes, there was a suggestion that the responses to genistein treatment varied among individuals, and most genistein target genes tended to cluster together based on similar expression patterns. While the exact molecular consequences of change in expression of each gene are undetermined, the stimulation of estrogen-responsive genes in an organ where estrogen is known to increase proliferation suggests a connection between soy isoflavones and increased cell proliferation. There are no comparable data on gene expression changes in breast epithelial samples from healthy women following a preventive intervention in the published literature, and the lack of significance in the between-group changes may relate to variability of gene expression over time in the control group. Notably, the low-density arrays included pre-and post-intervention samples of the same subject in the same array, and all PCR reactions were run at the end of the study within a single 3-week period.
We looked for signs of systemic estrogenicity (FSH, SHBG and estradiol) (33; 34). Estrogenic feedback will also decrease the plasma concentration of estradiol in postmenopausal women (35). None of these effects were observed in response to soy ingestion, suggesting that this combination of soy isoflavones does not cause a systemic estrogenic effect. Alternatively, breast epithelial gene expression and proliferation response may be a more sensitive indicator than these systemic measures.
Finally, although soy isoflavones were reliably detectable in the NAF of women in the soy group, there was no relationship between the presence of genistein in the NAF samples and Ki-67 indices in the breast. These analyses were limited since sufficient NAF yield at baseline and post intervention was achieved in 46 (47%) of women. A previous study suggested a pro-estrogenic effect on the breast based on pre and post-intervention measurements of TIFF1 in NAF samples (36). We did not measure TIFF1, but a number of other estrogen-related proteins (cathepsin-D, EGF, IGF-1) did not change significantly between groups following intervention.
We performed exploratory analyses focusing on the subset of women who displayed a plasma equol concentration of >5 ng/ml following intervention (27). There was a non-significant suggestion of a proliferative response in soy-treated premenopausal equol-producers, but our analyses were not powered for the sub-set of equol producers.
Notably, while soy isoflavone supplementation did not produce favorable biomarker modulation in the breast in the current study, the same agent in the same dose and schedule has produced favorable modulation of a different biomarker (MMP2) in a prostate cancertrial (37). This highlights the potential for organ-specificity of preventive agents. Our study also has important differences from the epidemiological data: dietary soy consumption occurs in smaller doses throughout the day, so that divided doses may have mimicked this pattern more closely. Secondly, we used a processed supplement whereas the epidemiologic studies of soy intake have examined intake of whole soy foods. Thirdly, soy exposure early in life may be necessary for beneficial effects (38). Thus future studies of processed soy supplements for breast cancer protection do not seem warranted, but investigations of soy food intake, particularly early in life are reasonable.
Supplementary Material
Figure 2.
Heatmap of gene expression by Taqman low density array in soy-treated women; clustering by gene group (Month 6 – Baseline Differences adjusted by housekeeping genes). Clustering is based on grouping ‘similar’ genes together, where ‘similarity’ here is in turn based on the degree of correlation of expressions levels among genes and among treatment groups. Pearson correlations were used to determine clustering.
Acknowledgments
Sponsor: NCI/Division of Cancer Prevention N01-CN-35157
Funding
This work was supported by the National Institutes of Health [N01-CN-35157to S.A.K.]. The authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
Footnotes
Clinical Trial Registration Number NCT00290758
Reference List
- 1.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 2.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 3.Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8(7):580–5. doi: 10.1007/s10434-001-0580-9. [DOI] [PubMed] [Google Scholar]
- 4.Tchou J, Hou N, Rademaker A, Jordan VC, Morrow M. Acceptance of tamoxifen chemoprevention by physicians and women at risk. Cancer. 2004;100(9):1800–6. doi: 10.1002/cncr.20205. [DOI] [PubMed] [Google Scholar]
- 5.Yen TW, Hunt KK, Mirza NQ, Thomas ES, Singletary SE, Babiera GV, et al. Physician recommendations regarding tamoxifen and patient utilization of tamoxifen after surgery for ductal carcinoma in situ. Cancer. 2004;100(5):942–9. doi: 10.1002/cncr.20085. [DOI] [PubMed] [Google Scholar]
- 6.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98(7):459–71. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 7.Dong JY, Qin LQ. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1270-8. [DOI] [PubMed] [Google Scholar]
- 8.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, et al. Soy food intake and breast cancer survival. JAMA. 2009;302(22):2437–43. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messina M, Hilakivi-Clarke L. Early intake appears to be the key to the proposed protective effects of soy intake against breast cancer. Nutr Cancer. 2009;61(6):792–8. doi: 10.1080/01635580903285015. [DOI] [PubMed] [Google Scholar]
- 10.Allred CD, Ju YH, Allred KF, Chang J, Helferich WG. Dietary genistin stimulates growth of estrogen-dependent breast cancer tumors similar to that observed with genistein. Carcinogenesis. 2001;22(10):1667–73. doi: 10.1093/carcin/22.10.1667. [DOI] [PubMed] [Google Scholar]
- 11.Allred CD, Allred KF, Ju YH, Clausen LM, Doerge DR, Schantz SL, et al. Dietary genistein results in larger MNU-induced, estrogen-dependent mammary tumors following ovariectomy of Sprague-Dawley rats. Carcinogenesis. 2004;25(2):211–8. doi: 10.1093/carcin/bgg198. [DOI] [PubMed] [Google Scholar]
- 12.Fabian CJ, Kimler BF, Zalles CM, Klemp JR, Kamel S, Zeiger S, et al. Short-Term Breast Cancer Prediction by Random Periareolar Fine-Needle Aspiration Cytology and the Gail Risk Model. J Natl Cancer Inst. 2000;92(15):1217–27. doi: 10.1093/jnci/92.15.1217. [DOI] [PubMed] [Google Scholar]
- 13.Mansoor I, Zalles C, Zahid F, Gossage K, Levenson RM, Rimm DL. Fine-needle aspiration of follicular adenoma versus parathyroid adenoma: the utility of multispectral imaging in differentiating lesions with subtle cytomorphologic differences. Cancer. 2008;114(1):22–6. doi: 10.1002/cncr.23252. [DOI] [PubMed] [Google Scholar]
- 14.Khan SA, Lankes HA, Patil DB, Bryk M, Hou N, Ivancic D, et al. Ductal lavage is an inefficient method of biomarker measurement in high-risk women. Cancer Prev Res (Phila) 2009;2(3):265–73. doi: 10.1158/1940-6207.CAPR-08-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masood S, Frykberg ER, McLellan GL, Scalapino MC, Mitchum DG, Bullard JB. Prospective evaluation of radiologically directed fine-needle aspiration biopsy of nonpalpable breast lesions. Cancer. 1990;66:1480–7. doi: 10.1002/1097-0142(19901001)66:7<1480::aid-cncr2820660708>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 16.The uniform approach to breast fine-needle aspiration biopsy. NIH Consensus Development Conference. Am J Surg. 1997;174(4):371–85. doi: 10.1016/s0002-9610(97)00119-0. [DOI] [PubMed] [Google Scholar]
- 17.Zalles C, Zalles N, Mahooti S, Zahid F, Khan SA, Rimm DL. Objective Spectral-Spatial Analysis of Random Periareolar Fine Needle Aspiration of Women at High Risk for Contralateral Breast Cancer. Breast Cancer Res Treat. 2009 Dec 13; Ref Type: Abstract. [Google Scholar]
- 18.Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269(2):226–42. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dip R, Lenz S, Antignac JP, Le BB, Gmuender H, Naegeli H. Global gene expression profiles induced by phytoestrogens in human breast cancer cells. Endocr Relat Cancer. 2008;15(1):161–73. doi: 10.1677/ERC-07-0252. [DOI] [PubMed] [Google Scholar]
- 20.Ma XJ, Salunga R, Tuggle JT, Gaudet J, Enright E, McQuary P, et al. Gene expression profiles of human breast cancer progression. Proc Natl Acad Sci U S A. 2003;100(10):5974–9. doi: 10.1073/pnas.0931261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gamache PH, Acworth IN. Analysis of phytoestrogens and polyphenols in plasma, tissue, and urine using HPLC with coulometric array detection. Proc Soc Exp Biol Med. 1998;217(3):274–80. doi: 10.3181/00379727-217-44232. [DOI] [PubMed] [Google Scholar]
- 22.Franke AA, Custer LJ. High-performance liquid chromatographic assay of isoflavonoids and coumestrol from human urine. J Chromatogr B Biomed Appl. 1994;662(1):47–60. doi: 10.1016/0378-4347(94)00390-4. [DOI] [PubMed] [Google Scholar]
- 23.Rotti K, Stevens J, Watson D, Longcope C. Estriol concentrations in plasma of normal, non-pregnant women. Steroids. 1975;25(6):807–16. doi: 10.1016/0039-128x(75)90045-8. [DOI] [PubMed] [Google Scholar]
- 24.Chatterton RT, Jr, Khan SA, Heinz R, Ivancic D, Lee O. Patterns of sex steroid hormones in nipple aspirate fluid during the menstrual cycle and after menopause in relation to serum concentrations. Cancer Epidemiol Biomarkers Prev. 2010;19(1):275–9. doi: 10.1158/1055-9965.EPI-09-0381. [DOI] [PubMed] [Google Scholar]
- 25.Chatterton RT, Jr, Geiger AS, Khan SA, Helenowski IB, Jovanovic BD, Gann PH. Variation in estradiol, estradiol precursors, and estrogen-related products in nipple aspirate fluid from normal premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13(6):928–35. [PubMed] [Google Scholar]
- 26.Maskarinec G, Watts K, Kagihara J, Hebshi SM, Franke AA. Urinary isoflavonoid excretion is similar after consuming soya milk and miso soup in Japanese-American women. Br J Nutr. 2008;100(2):424–9. doi: 10.1017/S0007114508898686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr. 2006;136(8):2188–93. doi: 10.1093/jn/136.8.2188. [DOI] [PubMed] [Google Scholar]
- 28.Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev. 2007;16(3):538–45. doi: 10.1158/1055-9965.EPI-06-0517. [DOI] [PubMed] [Google Scholar]
- 29.Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. Journal of Clinical Endocrinology and Metabolism. 1999;84(11):4017–24. doi: 10.1210/jcem.84.11.6152. [DOI] [PubMed] [Google Scholar]
- 30.Wood CE, Hester JM, Appt SE, Geisinger KR, Cline JM. Estrogen effects on epithelial proliferation and benign proliferative lesions in the postmenopausal primate mammary gland. Lab Invest. 2008;88(9):938–48. doi: 10.1038/labinvest.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood CE, Appt SE, Clarkson TB, Franke AA, Lees CJ, Doerge DR, et al. Effects of high-dose soy isoflavones and equol on reproductive tissues in female cynomolgus monkeys. Biol Reprod. 2006;75(3):477–86. doi: 10.1095/biolreprod.106.052142. [DOI] [PubMed] [Google Scholar]
- 32.Khan QJ, Kimler BF, Clark J, Metheny T, Zalles CM, Fabian CJ. Ki-67 expression in benign breast ductal cells obtained by random periareolar fine needle aspiration. Cancer Epidemiol Biomarkers Prev. 2005;14(4):786–9. doi: 10.1158/1055-9965.EPI-04-0239. [DOI] [PubMed] [Google Scholar]
- 33.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–75. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 34.Plymate SR, Moore DE, Cheng CY, Bardin CW, Southworth MB, Levinski MJ. Sex hormone-binding globulin changes during the menstrual cycle. J Clin Endocrinol Metab. 1985;61(5):993–6. doi: 10.1210/jcem-61-5-993. [DOI] [PubMed] [Google Scholar]
- 35.Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, Kurzer MS. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab. 1999;84(10):3479–84. doi: 10.1210/jcem.84.10.6067. [DOI] [PubMed] [Google Scholar]
- 36.Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, et al. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84(11):4017–24. doi: 10.1210/jcem.84.11.6152. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Ding Y, Catalona WJ, Yang XJ, Anderson WF, Jovanovic B, et al. MEK4function, genistein treatment, and invasion of human prostate cancer cells. J Natl Cancer Inst. 2009;101(16):1141–55. doi: 10.1093/jnci/djp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korde LA, Wu AH, Fears T, Nomura AM, West DW, Kolonel LN, et al. Childhood soy intake and breast cancer risk in Asian American women. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1050–9. doi: 10.1158/1055-9965.EPI-08-0405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.