Abstract
Introduction
Patients and mice with solid tumors, such as Lewis lung carcinoma (LLC), have defects in functions of immune effector cells. Endothelial cells, a component of the tumor vasculature, are potential regulators of immune cell functions. Therefore, these studies examined the impact of exposure to LLC tumor on the ability of endothelial cells to modulate immune cell functions.
Materials and methods
Endothelial cells were pre-treated with LLC tumor-conditioned medium (EndoT-sup) for 24 h. Control endothelial cells that were exposed to medium (EndoMedia) or epithelial cell-conditioned medium (EndoEpi-sup). After the initial 24 h incubation, endothelial cells were washed and fresh media was added. Cells were allowed to incubate for an additional 24 h. Supernatants from EndoMedia, EndoEpi-sup or EndoT-sup were collected and assayed for immune modulatory products and for immune modulatory activity.
Results
Supernatant from EndoT-sup contained increased levels of PGE2, IL-6 and VEGF as compared to EndoMedia and EndoEpi-sup controls. NK cell activity, as measured by TNF-α and IFN-γ secretion, was increased following exposure to media conditioned by EndoMedia and EndoEpi-sup. Exposure of NK cells to supernatants of EndoT-sup, also increases TNF-α and IFN-γ secretion, but to a lesser extent than by EndoMedia and EndoEpi-sup. Examination of macrophage functions demonstrated that supernatant from EndoT-sup decreased microbead phagocytosis and increased production of the immune suppressive mediators, IL-10 and PGE2. Lastly, T-cell responses to stimulation with anti-CD3 in the presence of supernatants from EndoT-sup were examined. IFN-γ production by CD8+ T-cells was reduced after exposure to EndoT-sup-conditioned medium, as compared to cells treatments with medium or control conditioned medium. Production of IFN-γ by CD4+ T-cells exposed to EndoT-sup was not altered.
Conclusions
Taken together, these studies demonstrate that tumors skew endothelial cells to disrupt NK cell, T-cell and macrophages functions, and represents a novel mechanism of tumor-induced immune suppression.
Keywords: Endothelial cell, Suppressor, Tumor, Immune suppression, Lewis lung carcinoma
Introduction
While the immune system is equipped to eliminate cancer cells, tumors possess numerous mechanisms by which they diminish anti-tumor immune responses. Defects in T-cell, NK cell and macrophage functions have been demonstrated in patients with solid tumors as well as tumor-bearing mice [7, 8, 11]. One process by which tumors suppress anti-tumor immune responses is by recruiting host cells to become immune suppressive. For example, tumor-associated fibroblasts (TAF) secrete high levels of the immune suppressant, transforming growth factor-β (TGF-β) [22]. Tumor-associated macrophages (TAM) represent another cell population that can destroy tumor cells but are instead induced to suppress anti-tumor immune responses by releasing elevated levels of the immune suppressive products, nitric oxide, IL-10 and TGF-β, and down-regulating levels of the immune stimulatory product IL-12 [10, 17, 14, 20]. An additional mechanism of tumor-induced immune suppression previously shown by our laboratory is the mobilization of suppressive CD34+ progenitor cells from the bone marrow by tumor secretion of high levels of granulocyte macrophage-colony stimulating factor (GM-CSF). These mobilized CD34+ cells diminish T-cell reactivity through the secretion of TGF-β [30].
In addition to being components of the vasculature, endothelial cells also serve as regulators of immune cell functions. Expression of programmed death-ligand 1 and 2 (PD-L1, PD-L2) by endothelial cells has been shown to down-regulate CD8+ T-cell activation and cytolysis [28]. Endothelial cell expression of PD-L1 induces the generation of CD4+CD25+Foxp3+ regulatory T-cells [15]. Liver sinusoidal endothelial cells have the capacity to present antigen from tumor cells and induce tumor-specific T-cell tolerance [2]. In addition to suppressing immune cell functions, endothelial cells are capable of stimulating immune cell functions. Co-culture experiments have demonstrated that endothelial cells stimulate IFN-γ production by CD8+ T-cells [3]. CD4+ T-cells co-cultured with endothelial cells have been shown to increase T-cell production of IL-2, IL-4 and IFN-γ in response to PHA stimulation [19].
While the role of endothelial cells as components of the tumor vasculature has been well studied, their ability to regulate immune cell functions in the tumor microenvironment remains unclear. Endothelial cells can secrete numerous immune suppressive products including vascular endothelial cell growth factor (VEGF), prostaglandin E2 (PGE2), TGF-β, IL-6 and IL-10 [16, 23, 26]. Conversely, they can also secrete the immune stimulatory product IL-12 [18]. The present studies demonstrate that supernatant from endothelial cells pretreated with tumor-conditioned medium (EndoT-sup) diminish NK cell, macrophage and T-cell functions as compared to supernatants from endothelial cells pretreated with either medium (EndoMedia) or with epithelial cell-conditioned medium (EndoEpi-sup) controls. These studies identify a novel mechanism by which tumors diminish host immune responses by skewing endothelial cells to disrupt NK cell, macrophage and T-cell functions.
Experimental methods
Mice
Eight to twelve week old, female C57BL/6 mice were obtained from Charles River (Wilmington, MA, USA). Mice were housed five to a cage with a 12/12 h light dark cycle and fed ad libitum. Mice were humanely euthanized by CO2 asphyxiation followed by cervical dislocation. Spleens and peritoneal macrophages were then collected and used for immunological analysis. All procedures were conducted with Institutional Animal Care and Use Committee approval.
Cell culture
Mouse bEnd.3 endothelial cells, MLE 12 epithelial cells, L929 fibroblast cells (ATCC, Manassas, VA, USA) and Lewis lung carcinoma (LCC) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated fetal bovine serum, 200 U/ml penicillin G, 200 μg/ml streptomycin sulfate, 500 μg/ml amphotericin B and 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA). Conditioned medium from MLE 12 mouse lung epithelial cells were used as controls since LLC cells are of lung epithelial cell origin.
Conditioned-media treatment and immune cell isolation
Media alone or 40% supernatants from subconfluent cultures of epithelial cells or LLC cells were used to treat subconfluent cultures of endothelial cells. After 24 h, endothelial cells were washed and fresh DMEM culture medium was added. Twenty-four hours later, endothelial cell-conditioned medium was collected and assayed by ELISA for the presence of immune regulatory products or used to treat freshly isolated immune cell populations.
T-cells were immunomagnetically isolated from the spleens of female C57BL/6 mice. Briefly, spleens were homogenized into a single cell suspension using a Stomacher 80 Biomaster to obtain a single cell suspension of spleen cells. After lysing red blood cells with ACK lysis buffer, spleen cells were washed three times and resuspended in separation buffer per the immunomagnetic column manufacturer’s instructions (Miltenyi Biotech Inc., Auburn, CA, USA). T-cells were isolated using a CD90+(Thy1.2+) immunomagnetic positive selection column. After isolation, T-cells were washed and plated in 96 well, round-bottom plates at a density of 2.5 × 105 cells per well on immobilized anti-CD3. T-cell purity was confirmed by flow cytometric analysis of CD3 expression and was determined to be 94% or higher.
NK cells were isolated using a negative selection magnetic bead column per the manufacturer’s instructions (Miltenyi Biotech Inc.) from the spleens of C57Bl/6 mice. After isolation, NK cells were washed three times and then plated in 96 well, round-bottom plates at a density of 2.5 × 105 cells per well. Both the isolated NK cells and T-cells were maintained in RPMI culture medium with 10% heat-inactivated fetal bovine serum, 200 U/ml penicillin G, 200 μg/ml streptomycin sulfate, 500 μg/ml amphotericin B, and 5 × 10−5 M 2-mercaptoethanol. Ten units/ml recombinant mouse IL-2 (R&D Systems, Minneapolis, MN, USA) was added to maintain cell viability. NK cell purity was determined to be 92% or higher as confirmed by immunostaining for the NK cell marker DX5.
Unstimulated macrophages were isolated from euthanized C57B1/6 mice by peritoneal lavage using 10 ml of ice-cold phosphate buffered saline (PBS). Peritoneal cells were washed, resuspended in phenol-free DMEM culture medium. Macrophages were plated in 24-well plates at a density of 5 × 105 cells per well. After allowing the macrophages to adhere 90 min, they were washed twice to remove non-adherent cells. Macrophage purity was confirmed by examination of cell morphology, adherence and phagocytic ability.
Immune function assays
After isolation and plating, macrophages, NK cells and T-cells were incubated with either medium alone or 40% EndoMedia, EndoEpi-sup or EndoT-sup for 24 h. Cells were washed and new medium was added. After an additional 24 h, supernatants from pretreated immune cells were collected for quantification of protein levels by ELISA and for assessment of immune modulatory effect as outlined in Fig. 1. Endothelial cell, T-cell, NK cell and macrophage secretion of immune regulatory factors was assessed by ELISA. These include measurement of PGE2 (Cayman Chemical, Ann Arbor, MI, USA), TNF-α (eBiosciences, San Diego, CA, USA), VEGF, MCP-1, TGF-β (R & D Systems, Minneapolis, MN, USA), IFN-γ, IL-2, IL-4, IL-6, IL-10 and IL-12 (BD Biosciences, San Jose, CA, USA). All ELISA’s were performed according to the manufacturer’s instructions.
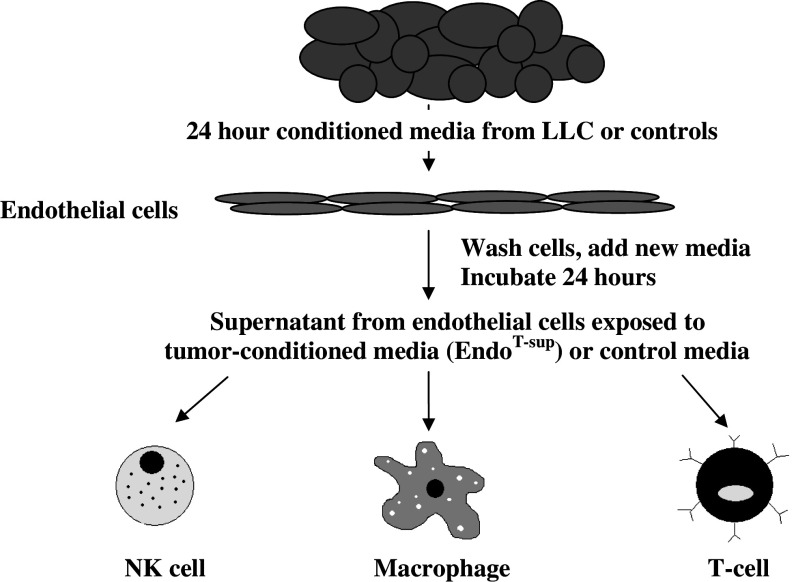
Fig. 1.
Experimental design: Cultured endothelial cells were treated with 40% LLC tumor-conditioned media for 24 h. Endothelial cells treated with media or lung epithelial cell-conditioned media served as controls. After the initial 24 h incubation, endothelial cells were washed and fresh media was added. Cells were allowed to incubate for an additional 24 h. Supernatants from EndoMedia, EndoEpi-sup or EndoT-sup were collected and assayed for immune modulatory products and for immune modulatory activity
Endothelial cell and T-cell proliferation was assessed by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) analysis (Promega). MTS is a tetrazolium compound that is reduced to formazan by metabolically active cells and was detected by measuring the absorbance at 492 nm.
In addition to measuring levels of IFN-γ, IL-10 and TNF-α that were secreted by T-cells, intracellular cytokine levels in CD4+ and CD8+ T-cells were measured by flow cytometry. All flow cytometry reagents were obtained from BD Biosciences. Prior to surface and intracellular staining, monensin (GolgiStop) was added to T-cells for 2 h according to the CytoStain Kit protocol. FcγII/III receptors were blocked with anti-CD16/CD32 monoclonal antibody. Cell surface antigen staining was performed using anti-CD4 and anti-CD8 monoclonal antibodies. After staining, cells were washed twice, then fixed and permeabilized with Cytofix/Cytoperm. Marker channels were set using the antibody isotype controls. Four-color flow cytometric analysis was performed using BD FACSCanto Flow cytometer with FACS Diva flow cytometry analysis software.
Macrophage activation was assessed by secretion of TNF-α and IL-10 as well as phagocytosis of fluorescent microbeads. To measure macrophage phagocytosis, 10 μl of 1:100 diluted FITC polymer microspheres (Duke Scientific Corporation, Palo Alta, CA, USA) were added to macrophages for 4 h at 37°C. Following treatments, the cells were washed three times and resuspended in PBS. To confirm that bead uptake was a result of phagocytosis, control cells were incubated with beads at 0°C. Following microscopic examination, macrophages were detached from the plates by scraping and bead phagocytosis was quantified by flow cytometric analysis.
Statistical analysis
Statistical analyses was conducted using GraphPad Prism 4.03. ANOVA analysis with post hoc student t test was used to calculate statistical significance between experimental treatment and each of the control treatments. Data shown are mean values of multiple experiments. Microphotographs, dot plots and histograms are representative results of multiple experiments.
Results
Endothelial cells treated with tumor-conditioned media secrete increased levels of immune suppressive factors and diminished levels of immune stimulatory factors
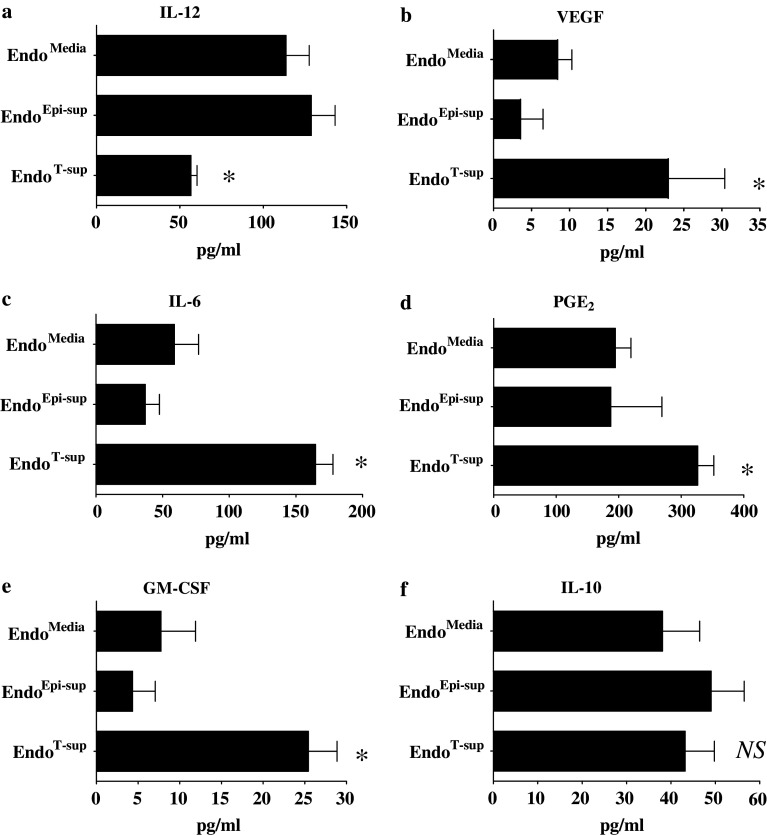
Endothelial cells secrete numerous immune modulatory factors such as IL-12, VEGF, IL-6, PGE2 and GM-CSF [18, 16, 23, 26]. Therefore, we examined if exposure to tumor-secreted products alters endothelial cell production of these immune modulators. The immune stimulatory product IL-12 was found to be secreted at lower levels by EndoT-sup than by EndoMedia (P = 0.0073) or EndoEpi-sup (P = 0.0028) (Fig. 2a).
Fig. 2.
Tumor-conditioned media alters endothelial cell secretion of immune modulatory products: Endothelial cell secretion of the immune stimulatory factor, IL-12, and potentially immune inhibitory factors, VEGF, PGE2, GM-CSF, IL-6 and IL-10 was examined by ELISA after pretreatment with tumor-conditioned media or controls. * indicates P ≤ 0.008 or less compared to EndoMedia or EndoEpi-sup. NS indicates no significant difference between treatment groups. All data are means ± SEM with n = 6 or more for all treatment groups
Secretion of immune suppressive products by endothelial cells in response to tumor-conditioned media exposure was also examined. While VEGF has been shown to have numerous pro-angiogenic properties, it has also been shown to inhibit functional dendritic cell maturation and inhibit T-cell development [1, 2]. Significant increases in VEGF secretion were observed in EndoT-sup as compared to EndoMedia (P = 0.0051) or EndoEpi-sup (P = 0.0006) (Fig. 2b). Levels of IL-6 secreted by EndoT-sup were increased as compared to levels secreted by EndoMedia or EndoEpi-sup (P = 0.0008 and P < 0.0001 respectively) (Fig. 2c). Levels of PGE2 secreted by EndoT-sup were increased as compared to either EndoMedia (P = 0.0044) or EndoEpi-sup controls (P = 0.0025) (Fig. 2d). Lastly, secretion of the immune modulator GM-CSF was examined. EndoT-sup secreted significantly higher levels than EndoMedia or EndoEpi-sup controls (P = 0.0082 and P = 0.007, respectively) (Fig. 2e). Endothelial cell secretion of IL-10 was measured and was not affected by exposure to tumor-conditioned media (Fig. 2f). Secretion of TGF-β was also not altered by treatment with epithelial or tumor conditioned media exposure (data not shown). No significant differences were observed in the levels of immune regulatory mediators secreted by EndoMedia and EndoEpi-sup control groups, indicating that alterations in secretion were selective to tumor-conditioned media treatment. To confirm that these results were specific to endothelial cells, fibroblasts were treated with tumor-conditioned media or controls and secretion of immune modulatory factors was examined. We observed no significant alterations in fibroblast secretion of PGE2, VEGF, GM-CSF or IL-12 in response to tumor-conditioned medium compared to treatments with medium alone or epithelial cell-conditioned media (data not shown). These results demonstrate that tumor-secreted products induce endothelial cells to increase secretion of immune suppressive products while down-regulating secretion of immune stimulatory products.
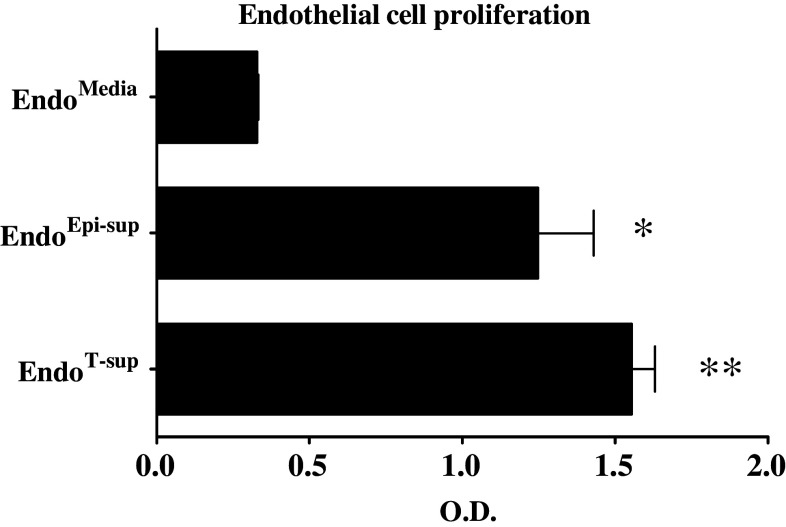
Endothelial cell proliferation is increased after exposure to epithelial cell-conditioned media or tumor-conditioned media
Endothelial cell proliferation was assessed by MTS after exposure to tumor-conditioned media or control treatments to examine if skewed endothelial cell production of immune modulatory products was influenced by their proliferation. Exposure of endothelial cells to epithelial cell-conditioned media and tumor-conditioned medium both increased endothelial cell proliferation (Fig. 3) when compared to endothelial cells treated with media alone (P < 0.0001). Differences in proliferation between EndoEpi-sup and EndoT-sup endothelial cells was minimal, but statistically significant (P < 0.0001). These results demonstrate that differences in immune mediator production by endothelial cells are not the result of differences in endothelial cell proliferation. EndoMedia and EndoEpi-sup have no significant difference in their secretion of immune modulatory products, yet have substantial differences in proliferation rates. Furthermore, EndoEpi-sup and EndoT-sup have similar levels of proliferation, yet altered production of immune modulatory products.
Fig. 3.
Epithelial cell-conditioned media and tumor-conditioned media increase endothelial cell proliferation: Endothelial cell proliferation in response to 24 h exposure to conditioned media treatment was examined by MTS. * indicates P < 0.0001 when compared to EndoMedia control. ** indicates P < 0.0001 compared to EndoMedia or EndoEpi-sup. All data are means ± SEM with n ≥ 4 for all treatment groups
Treatment with media conditioned by EndoMedia and EndoEpi-sup is stimulatory to NK cells, while media conditioned by EndoT-sup has reduced stimulatory capacity
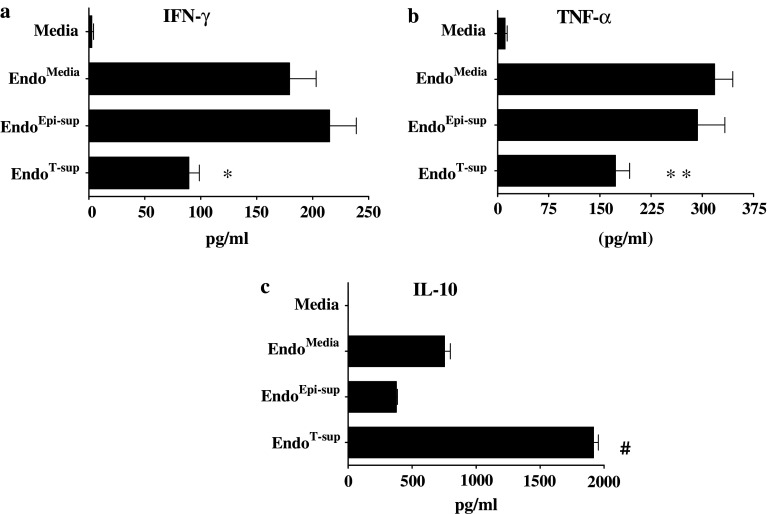
After observing that exposure to tumor-conditioned media altered endothelial cell secretion of immune regulatory products, studies were conducted to determine the effects of endothelial cell-conditioned media on NK cell functions. After isolation, NK cells were incubated with media alone or media conditioned by EndoMedia, EndoEpi-sup or EndoT-sup. NK cells were then washed, fresh media added and the cells allowed to incubate for an additional 24 h. Media conditioned by these NK cells was then used to quantitative levels of secreted IFN-γ and TNF-α as a measure of NK cell activation [25]. NK cell exposure to media alone resulted in low or undetectable levels of IFN-γ and TNF-α (Fig. 4a, b). However, when treated with media conditioned by EndoMedia or EndoEpi-sup, NK cell secretion of IFN-γ and TNF-α was significantly increased (P < 0.0001 for both cytokines). However, treatment of NK cells with media conditioned by EndoT-sup resulted in decreased levels of IFN-γ secretion compared to secretion levels after treatment with media from EndoMedia (P = 0.0057) or EndoEpi-sup (P = 0.0006) cells. Similar effects were observed for TNF-α secretion as were seen for IFN-γ secretion. NK cell secretion of TNF-α was significantly increased when exposed to media conditioned by EndoMedia or EndoEpi-sup compared to treatment with media alone (P < 0.0001 and P = 0.0005, respectively). NK cells treated with media conditioned by EndoT-sup demonstrated reduced TNF-α levels as compared to NK cells treated by conditioned medium from EndoMedia or EndoEpi-sup controls (P = 0.0018 and P = 0.0215, respectively).
Fig. 4.
EndoMedia and EndoEpi-sup strongly stimulate NK cell secretion of IFN-γ and TNF-α, while EndoT-sup stimulates IL-10 production: Secretion of IFN-γ, TNF-α and IL-10 by NK cells was measured to assess their responses to EndoT-sup. Immunomagnetically-isolated, splenic NK cells that were cultured for 24 h with EndoT-sup or controls, washed and then cultured for an additional 24 h. Levels of IFN-γ, TNF-α and IL-10 in the conditioned media were assessed by ELISA. * indicates P ≤ 0.005 compared to each control group. ** indicates P ≤ 0.002 compared to all each treatment group. # indicates P < 0.0001 compared each control group. All data represent means ± SEM with an n = 4 for all treatment groups
NK cell secretion of the immune suppressive cytokine, IL-10, following exposure to endothelial cell-conditioned media was also examined. Secretion of IL-10 by NK cells was moderately upregulated following treatment with media conditioned by EndoMedia or EndoEpi-sup compared to treatment with media alone. When NK cells were treated with media from EndoT-sup, IL-10 secretion was significantly enhanced compared to NK cells treated with media from EndoMedia and EndoEpi-sup controls (P < 0.0001) (Fig. 4c). These results indicate that endothelial cell-secreted products can stimulate NK cell activation to produce the immune stimulatory cytokines IFN-γ and TNF-α. However, pretreatment of endothelial cells with tumor-conditioned media disrupts their ability to produce stimulatory cytokines and instead induces their secretion of IL-10.
Treatment with medium-conditioned by EndoT-sup diminishes macrophage phagocytosis and increases their production of immune suppressive mediators
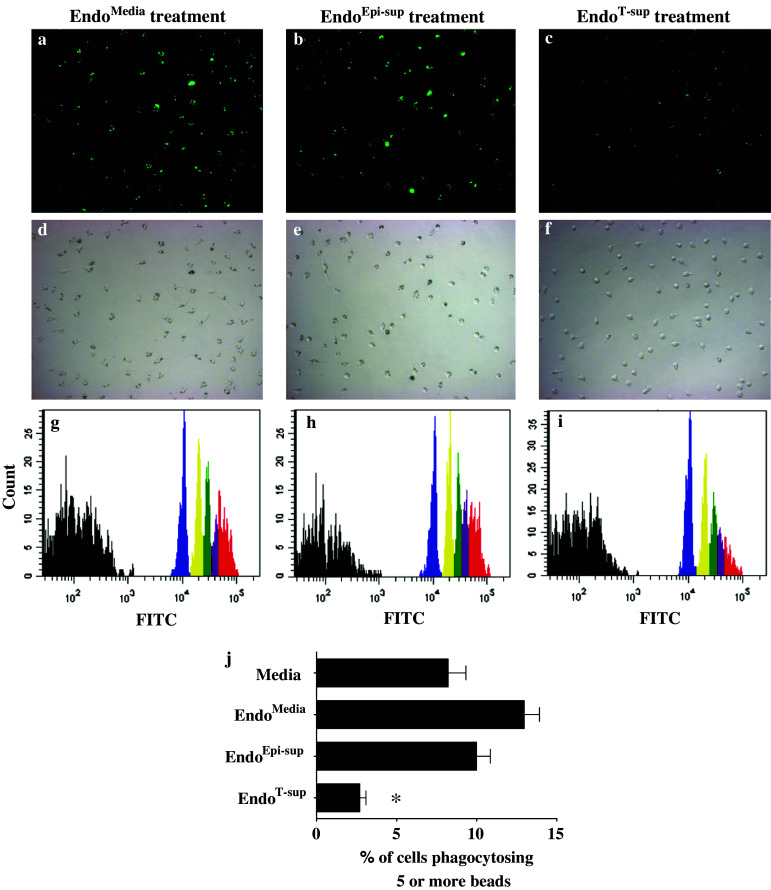
In addition to NK cell studies, the effects of conditioned medium from EndoT-sup on macrophage functions were also examined. Macrophages were treated for 24 h with medium alone or with EndoMedia, EndoEpi-sup or EndoT-sup conditioned media, then washed and allowed to incubate for an additional 24 h. Phagocytosis was measured using a fluorescent microbead uptake assay and was quantified by flow cytometric analyses. When macrophages were treated with media conditioned by EndoMedia or EndoEpi-sup, phagocytosis of five or more beads was slightly elevated when compared to macrophages that were incubated in medium alone (Fig. 5). However, macrophages that were incubated with conditioned medium from EndoT-sup displayed a significant reduction in phagocytosis of five or more beads (Fig. 5) compared to those treated with control conditioned media (P < 0.0001 for all control groups). Treatment with EndoT-sup-conditioned medium also reduced total macrophage bead phagocytosis and phagocytosis of two, three or four beads compared to each of the control treatment groups (data not shown).
Fig. 5.
Treatment with EndoT-sup diminishes macrophage phagocytosis of fluorescent microbead: Macrophage phagocytosis was assessed by their uptake of fluorescent microbeads. Bead phagocytosis was assessed microscopically and by flow cytometric analysis. a–c Representative fluorescent photographs of macrophages after 4 h incubation with fluorescent beads. d–f Bright field images of panels (a–c) to demonstrate equal distribution of macrophages. g–i Representative histograms of flow cytometric analysis of microbead phagocytosis. The percent of macrophages phagocytosing one bead is depicted in blue, those taking up two beads in yellow, three beads in green and four beads is shown in purple. The percent of macrophages phagocytosing five or more beads is represented in the red peaks. j Mean percent ± SEM of flow cytometric quantification of macrophage phagocytosis of five or more beads including the data represented in panels (a–i) * indicates P < 0.0001. n = 5 for all treatment groups
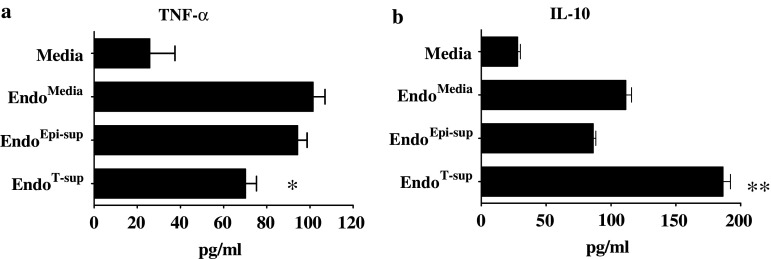
In addition to phagocytosis, secretion of TNF-α and IL-10 was also measured to assess the effects of endothelial cell-conditioned media on macrophage functions. Treatment with media conditioned by EndoMedia or EndoEpi-sup stimulated macrophage TNF-α secretion as compared to that secreted by macrophages treated with medium alone (P < 0.0001 for both controls). Treatment with medium conditioned by EndoT-sup reduced TNF-α secretion compared to macrophages treated with conditioned media from EndoMedia or EndoEpi-sup (P = 0.002 and P = 0.001 respectively) (Fig. 6a). Macrophages that were treated in media conditioned by EndoMedia or EndoEpi-sup secreted moderately increased levels of IL-10 as compared to treatment with medium alone (P < 0.0001 for both controls) (Fig. 6b). However, when treated with medium conditioned by EndoT-sup, there was a significant increase in macrophage IL-10 secretion compared to the secretion by all other control treatments (P < 0.0001). These results indicate that tumor-conditioned medium skews endothelial cells to secrete products that suppress macrophage phagocytosis and increase macrophage production of immune suppressive products.
Fig. 6.
Assessment of cytokine secretion by macrophages in response to conditioned media from treated endothelial cells: Peritoneal macrophages were cultured for 24 h with EndoT-sup or controls, washed and then cultured for an additional 24 h. Conditioned media was then collected and assessed by ELISA for the presence of TNF-α and IL-10. * indicates P ≤ 0.002 compared to each control group. ** indicates P < 0.0001 compared to each control group. All data are means ± SEM with n = 4 for all treatment groups
Treatment with medium-conditioned by EndoT-sup diminishes CD8+ T-cell responses to anti-CD3 stimulation, but does not alter CD4+ T-cell responses
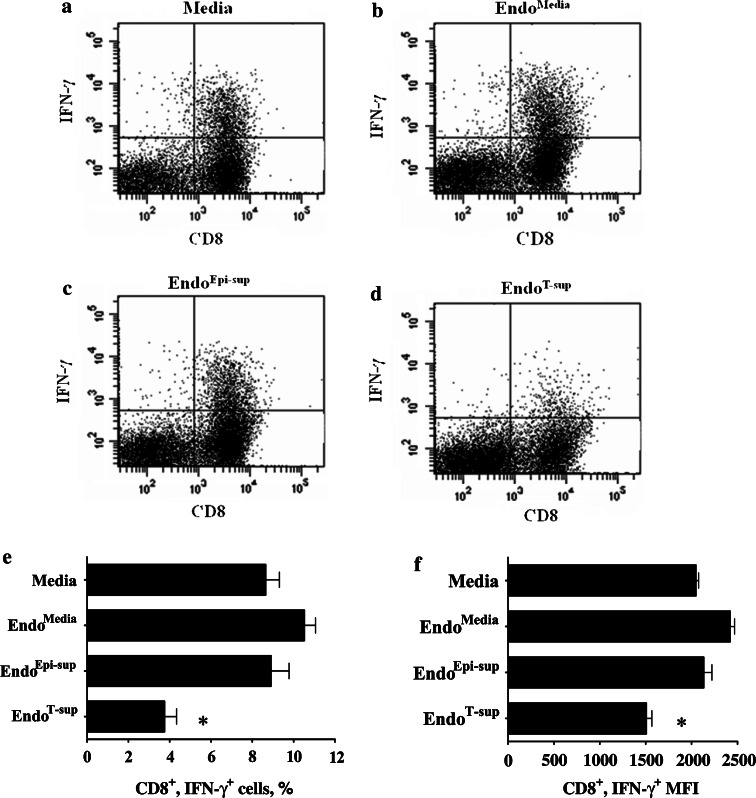
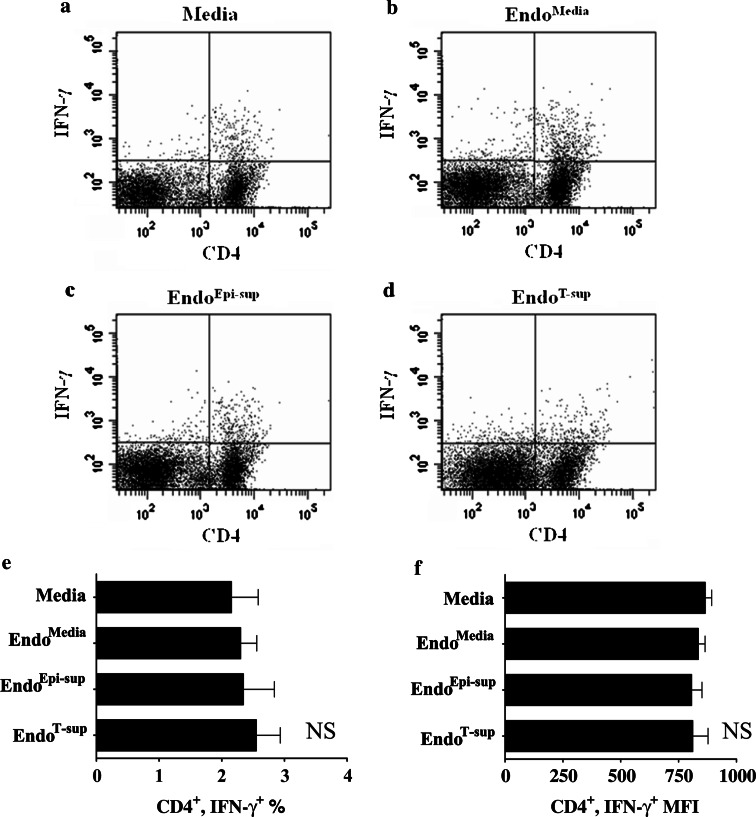
The impact of endothelial cell supernatants on T-cell functions was next examined. CD90+ T-cells were plated on anti-CD3-coated, 96-well plates and treated with medium alone or media conditioned by EndoMedia, EndoEpi-sup or EndoT-sup. Flow cytometric analysis demonstrated that when T-cells were treated with EndoT-sup-conditioned medium, there was a significant reduction in the proportion of CD8+ T-cells that stained positive for IFN-γ as compared to cultures that were treated with medium only or media conditioned by EndoMedia or EndoEpi-sup controls (P < 0.0001) (Fig. 7a–e). The mean fluorescent intensity of IFN-γ+ staining was also reduced in CD8+ T-cells (P < 0.0001) (Fig. 7f). The percent of CD4+ T-cells that stained positive for IFN-γ was not affected by treatment with media conditioned by EndoMedia, EndoEpi-sup or EndoT-sup (Fig. 8a–e). Mean fluorescent intensity of IFN-γ staining by CD4+ T-cells also did not change significantly with any of the endothelial cell-conditioned media treatments (Fig. 8f).
Fig. 7.
CD8+ T-cell responses to anti-CD3 stimulation in the presence of conditioned media from endothelial cells: CD8+ T-cell activation was assessed by flow cytometric determination of intracellular IFN-γ staining. a–d Representative data of T-cells that were immunostained for CD8 and IFN-γ. e Mean percent of CD8+/IFN-γ+ ± SEM with n ≥ 4 for all treatments groups. f Mean fluorescent intensity (MFI) of flow cytometric staining for IFN-γ by CD8+ T-cell ±SEM with n ≥ 4 for all treatments groups. * indicates P < 0.0001 compared to each control group
Fig. 8.
CD4+ T-cell responses to anti-CD3 stimulation in the presence of conditioned media from endothelial cells: CD4+ T-cell production of IFN-γ was assessed by intracellular flow cytometric analysis. a–d Representative data of CD4+ T-cells also staining positive for IFN-γ. e Mean percent of CD4+/IFN-γ+ ± SEM with n ≥ 4 per treatment group. f MFI of flow cytometric staining for IFN-γ by CD4+ T-cells ± SEM with n ≥ 4 per treatment group. NS indicates there is significant difference between any of the treatment groups
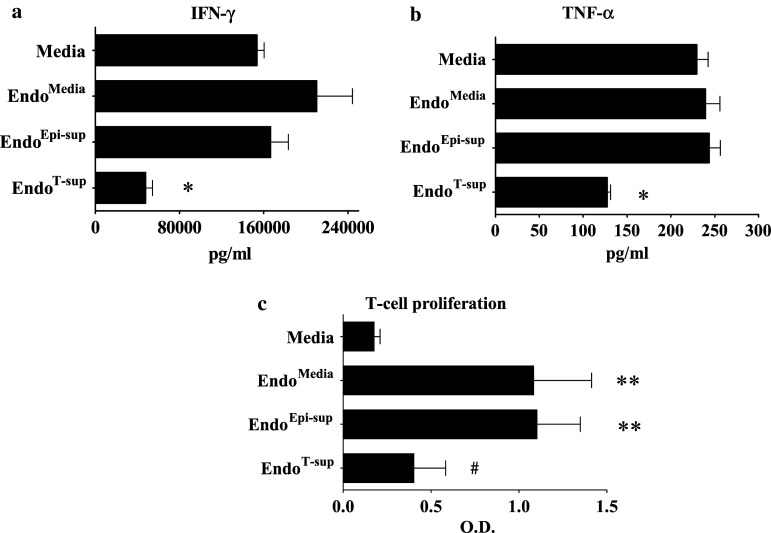
Total levels of secreted IFN-γ were also assessed by ELISA to confirm the alterations in IFN-γ production that were observed by intracellular cytokine staining. Similar to the intracellular IFN-γ levels, treatment of T-cells with medium conditioned by EndoT-sup resulted in a significant reduction of IFN-γ secretion compared to that from T-cells treated with medium only (P < 0.0001), medium conditioned by EndoMedia (P = 0.0002) or medium conditioned by EndoEpi-sup (P < 0.0001) (Fig. 9a). Levels of TNF-α secretion by T-cells were similar among all control treatment groups, but were reduced by half following treatment with EndoT-sup-conditioned medium (P < 0.0001) (Fig. 9b). When unstimulated T-cells were treated with endothelial cell supernatants, there were no significant difference in cytokine production by T-cells that were treated with media conditioned by EndoT-sup or the other control endothelial cell supernatants (data not shown). Also examined were the effects of endothelial cell conditioned media on T-cell proliferation. Both conditioned medium from EndoMedia and EndoEpi-sup increased T-cell proliferation compared to treatment with media alone (P < 0.0001 for both controls) (Fig. 9c). When T-cells were exposed to media from EndoT-sup, proliferation was diminished compared to medium conditioned by EndoMedia (P = 0.0003) and EndoEpi-sup (P < 0.0001). Levels of T-cell proliferation were similar between T-cells treated with conditioned media from EndoT-sup and media alone. While proliferation levels were similar between media alone conditioned media from EndoT-sup treatment, TNF-α and IFN-γ production was reduced in T-cells treated with conditioned media from EndoT-sup versus media treatment alone (Fig. 9a, b). These results indicate that tumor-secreted products skew endothelial cells to diminish T-cell activation responses. In addition, flow cytometric analysis of IFN-γ production suggests that the suppressive effects of EndoT-sup-conditioned media may be limited to CD8+ T-cell responses.
Fig. 9.
Assessment of T-cell inflammatory cytokine production and proliferation in response to anti-CD3 stimulation: CD90+ T-cell secretion of TNF-α and IFN-γ was examined by ELISA. Mean levels ± SEM of IFN-γ and TNF-α following exposure to conditioned medium for EndoT-sup are in (a) and (b) respectively. * indicates P ≤ 0.0002 compared to each control treatment group. c T-cell proliferation in response to endothelial cell-conditioned media exposure was assessed by MTS. Data is mean OD ± SEM with n = 5. ** indicates P < 0.0001 compared to treatment with media alone. # indicates P ≤ 0.0003 when compared to EndoMedia and EndoEpi-sup controls
Discussion
Tumors manipulate normal cell populations with in the tumor microenvironment to aid in suppressing anti-tumor immune responses. For example, tumors also secrete products that skew monocytes and dendritic cells to increase production of Th2 cytokines, thereby reducing Th1 anti-tumor reactivity [29]. Tumors selectively recruit CD4+CD25+Foxp3+ cells to suppress tumor-specific T-cell responses [4]. Furthermore, tumor-associated macrophages secrete products, such as TGF-β and IL-10 that suppress anti-tumor immune responses [14, 20, 10]. Tumor-associated fibroblasts have also been implicated as suppressors of tumor-associated T-cell functions [22]. While endothelial cells have been shown to be contact-dependent regulators of immune cell functions [5], few studies have examined their role in modulating immune functions in the tumor microenvironment. We hypothesized that, due to their ability to secrete numerous immune regulatory products, endothelial cells are manipulated by tumors to suppress immune cell functions. In the present studies, we have shown that tumor-secreted products skew endothelial cells to increase production of immune suppressive mediators and disrupt NK cell, T-cell and macrophage functions.
In our in vitro model, it was demonstrated that tumor-secreted products skew endothelial cells to disrupt NK cell, macrophage and T-cell functions. Several endothelial cell-derived factors were examined due to their ability to disrupt immune cell functions. While VEGF has been shown to have numerous pro-angiogenic properties, it has also been shown to inhibit T-cell development and is involved in tumor-induced immune suppression [24]. IL-6 was examined due to its ability to inhibit Th1 polarization and promote Th2 differentiation of T-cells, thereby diminishing anti-tumor T-cell responses [6]. Lastly, PGE2 was examined due to its ability to inhibit T-cell functions and down regulate IL-12 production [21, 27]. When endothelial cells were treated with tumor-conditioned media, their production of VEGF-A, IL-6, GM-CSF and PGE2 increased compared to the levels produced by control endothelial cells. Conversely, endothelial cell secretion of the immune stimulatory product, IL-12, was decreased in response to treatment with tumor-conditioned medium. Analysis of IL-10 and TGF-β secretion revealed no significant difference in production by endothelial cells that were exposed to tumor-conditioned media or control treatments. These studies also demonstrated that tumor-conditioned media skews endothelial cells to diminish T-cell, NK cell and macrophage functions.
Several studies have examined endothelial cells’ ability to regulate T-cell functions, though most have focused on contact-dependent regulation. Briscoe et al. demonstrated that co-culture of T-cells with endothelial cells enhances CD4+ T-cell IFN-γ production in vitro [3]. However, our studies did not demonstrate any alterations in CD4+ T-cell IFN-γ production following exposure to media from EndoT-sup. One reason for this may be that contact between T-cells and endothelial cells was required in these studies, whereas our model was contact independent. Work by Katz et al. [12] also examined T-cell/endothelial cells interactions using a liver sinusoidal endothelial cell model. Their study observed that endothelial cells alone were insufficient to stimulate T-cell responses. These studies focused on liver sinusoidal endothelial cells ability to stimulate allogeneic or antigen specific T-cell responses, whereas our studies focused on the tumor skewing of endothelial cells to disrupt T-cells responses to anti-CD3 stimulation. Our studies did not investigate the effects of tumor skewing on endothelial cell presentation of antigen to stimulate T-cell responses.
Previous studies focusing on endothelial cell modulation of NK cell and macrophage functions have predominantly examined contact-dependent regulation. Limited work has focused on contact-independent activation of NK cells by endothelial cells. Endothelial cell and monocyte/macrophage interactions have also been investigated including studies focusing on post-hemorrhagic vasospasm, Mycobacterium leprae and other physiological responses involving inflammation [1, 9, 13]. The majority of this work has focused on endothelial cell expression of adhesion molecules and their effects on macrophage functions. These studies did not, however, examine the immune regulatory effects of endothelial cells in the tumor microenvironment. Studies are currently ongoing to determine what factor(s), including IL-12 and IL-12 family members, are responsible for endothelial cell modulation of NK cell functions.
The studies presented here demonstrate that normal endothelial cells have immune stimulatory effects, whereas tumor-skewed endothelial cells suppress these stimulatory effects. This is analogous to the Th1 and Th2 balance which is skewed toward Th2 in tumor-bearers. By analogy, tumor-exposed endothelial cells could be termed to have an E2 phenotype as compared to epithelial cell-exposed endothelial cells, which could be termed to have an E1 phenotype. One area not addressed by these studies is whether anti-tumor immune responses are suppressed by tumor-exposed endothelial cells. Studies are currently ongoing to assess the effects of endothelial cells in suppressing NK cell and T-cell reactivity against tumor cells. Studies are also currently underway to determine the tumor-secreted factor(s) responsible for skewing endothelial cells toward an E2 phenotype. Another question that remains to be answered is how tumor-exposed endothelial cells are disrupting immune cell functions, though several candidate mediators have been identified. Studies are underway to examine the contribution of E2 endothelial cells in tumor-induced immune suppression in vivo. The in vitro studies conducted thus far identify a novel mechanism of tumor-induced immune suppression through the induction of tumor-induced E2 endothelial cells.
Acknowledgments
This work was supported by the Research Service of the Department of Veterans Affairs and by grants R01CA85266 and R01CA97813 from the National Institutes of Health to MRIY.
References
- 1.Barker LP. Mycobacterium leprae interactions with the host cell: recent advances. Indian J Med Res. 2006;123:748–759. [PubMed] [Google Scholar]
- 2.Berg M, Wingender G, Djandji D, Hegenbarth S, Momburg F, Hammerling G, Limmer A, Knolle P. Cross-presentation of antigens from apoptotic tumor cells by liver sinusoidal endothelial cells leads to tumor-specific CD8+ T cell tolerance. Eur J Immunol. 2006;36:2960–2970. doi: 10.1002/eji.200636033. [DOI] [PubMed] [Google Scholar]
- 3.Briscoe DM, Henault LE, Geehan C, Alexander SI, Lichtman AH. Human endothelial cell costimulation of T cell IFN-gamma production. J Immunol. 1997;159:3247–3256. [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 1997;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Danese S, Dejana E, Fiocchi C. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178:6017–6022. doi: 10.4049/jimmunol.178.10.6017. [DOI] [PubMed] [Google Scholar]
- 6.Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39(9):531–536. doi: 10.1016/S0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 7.Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol. 1998;64:275–290. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- 8.Finke J, Ferrone S, Frey A, Mufson A, Ochoa A. Where have all the T cells gone? Mechanisms of immune evasion by tumors. Immunol Today. 1999;20:158–160. doi: 10.1016/S0167-5699(98)01435-2. [DOI] [PubMed] [Google Scholar]
- 9.Gallia GL, Tamargo RJ. Leukocyte-endothelial cell interactions in chronic vasospasm after subarachnoid hemorrhage. Neurol Res. 2006;28:750–758. doi: 10.1179/016164106X152025. [DOI] [PubMed] [Google Scholar]
- 10.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:177–189. doi: 10.1023/A:1020304003704. [DOI] [PubMed] [Google Scholar]
- 11.Lizee G, Radvanyi LG, Overwijk WW, Hwu P. Improving antitumor immune responses by circumventing immunoregulatory. Cells Mech. 2006;12:4794–4803. doi: 10.1158/1078-0432.CCR-06-0944. [DOI] [PubMed] [Google Scholar]
- 12.Katz SC, Pillarisetty VG, Bleier JI, Shah AB, DeMatteo RP. Liver sinusoidal endothelial cells are insufficient to activate T cells. J Immunol. 2004;173:230–235. doi: 10.4049/jimmunol.173.1.230. [DOI] [PubMed] [Google Scholar]
- 13.Kindle L, Rothe L, Kriss M, Osdoby P, Collin-Osdoby P. Human microvascular endothelial cell activation by IL-1 and TNF-α stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional. Osteoclasts. 2006;21:193–206. doi: 10.1359/JBMR.051027. [DOI] [PubMed] [Google Scholar]
- 14.Konur A, Kreutz M, Knuchel R, Krause SW, Andreesen R. Three-dimensional co-culture of human monocytes and macrophages with tumor cells: analysis of macrophage differentiation and activation. Int J Cancer. 1996;66:645–652. doi: 10.1002/(SICI)1097-0215(19960529)66:5<645::AID-IJC11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Krupnick AS, Gelman AE, Barchet W, Richardson S, Kreisel FH, Turka LA, Colonna M, Patterson GA, Kreisel D. Murine vascular endothelium activates and induces the generation of allogeneic CD4 + 25+Foxp3+ regulatory T cells. J Immunol. 2005;175:6265–6270. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 16.Lefkowitz DL, Roberts E, Grattendick K, Schwab C, Stuart R, Lincoln J, Allen RC, Moguilevsky N, Bollen A, Lefkowitz SS. The endothelium and cytokine secretion: the role of peroxidases as immunoregulators. Cell Immunol. 2000;202(1):23–30. doi: 10.1006/cimm.2000.1638. [DOI] [PubMed] [Google Scholar]
- 17.Lejeune P, Lagadec P, Onier N, Pinard D, Ohshima H, Jeannin JF. Nitric oxide involvement in tumor-induced immunosuppression. J Immunol. 1994;152:5077–5083. [PubMed] [Google Scholar]
- 18.Lienenluke B, Germann T, Kroczek RA, Hecker M. CD154 stimulation of interleukin-12 synthesis in human endothelial cells. Eur J Immunol. 2000;30:2864–2870. doi: 10.1002/1521-4141(200010)30:10<2864::AID-IMMU2864>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 19.Ma W, Pober JS. Human endothelial cells effectively costimulate cytokine production by, but not differentiation of, naive CD4+ T cells. J Immunol. 1998;161:2158–2167. [PubMed] [Google Scholar]
- 20.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25(3):315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuhashi M, Liu J, Cao S, Shi X, Ma X. Regulation of interleukin-12 gene expression and its anti-tumor activities by prostaglandin E2 derived from mammary carcinomas. J Leulock Biol. 2004;76(2):322–332. doi: 10.1189/jlb.1203641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Jr, Yokota SJ, Bankert RB. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol. 2007;178:5552–5562. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 23.Ohira H, Abe K, Yokokawa J, Takiguchi J, Rai T, Shishido S, Sato Y. Adhesion molecules and CXC chemokines in endotoxin-induced liver injury. Fukushima J Med Sci. 2003;49:1–13. doi: 10.5387/fms.49.1. [DOI] [PubMed] [Google Scholar]
- 24.Ohm JE, Gabrilovich DI, Sempowski GD, Sempowski E, Parman KS, Nadaf S, Carbone DP. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101:4878–4886. doi: 10.1182/blood-2002-07-1956. [DOI] [PubMed] [Google Scholar]
- 25.Papamichail M, Perez S, Gritzapis A, Baxevanis C. Natural killer lymphocytes: biology, development and function. Cancer Immunol Immunother. 2004;53:176–186. doi: 10.1007/s00262-003-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirtskhalaishvili G, Nelson JB. Endothelium-derived factors as paracrine mediators of prostate cancer progression. Prostate. 2000;44:77–87. doi: 10.1002/1097-0045(20000615)44:1<77::AID-PROS10>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hernandez JL, Gendler SJ, Mukherjee P. Reduced T-cell and dendritic cell function is related to cyclooxygenase-2 overexpression and prostaglandin E2 secretion in patients with breast cancer. Ann Surg Oncol. 2004;11(3):328–339. doi: 10.1245/ASO.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 29.Young MR. Trials and tribulations of immunotherapy as a treatment option for patients with squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 2004;53:375–382. doi: 10.1007/s00262-003-0456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young MR, Wright MA, Matthews JP, Malik I, Prechel M. Suppression of T cell proliferation by tumor-induced granulocyte-macrophage progenitor cells producing transforming growth factor-beta and nitric oxide. J Immunol. 1996;156:1916–1922. [PubMed] [Google Scholar]