Abstract
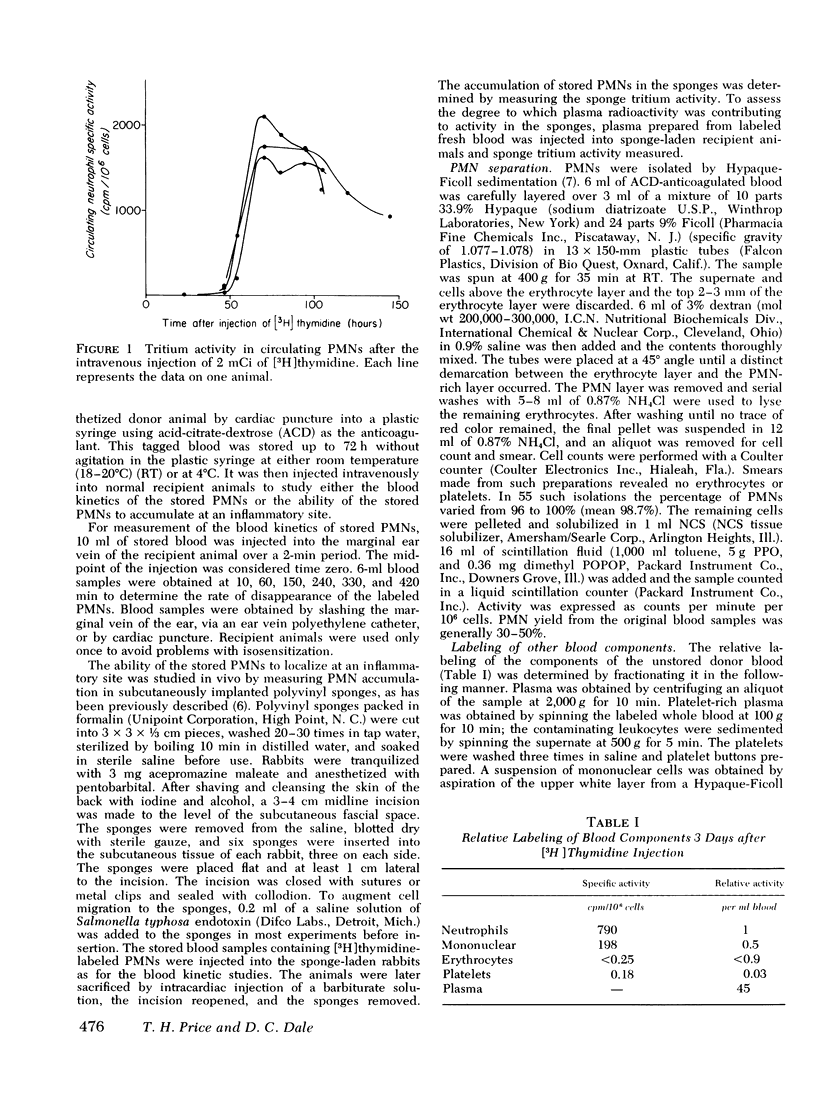
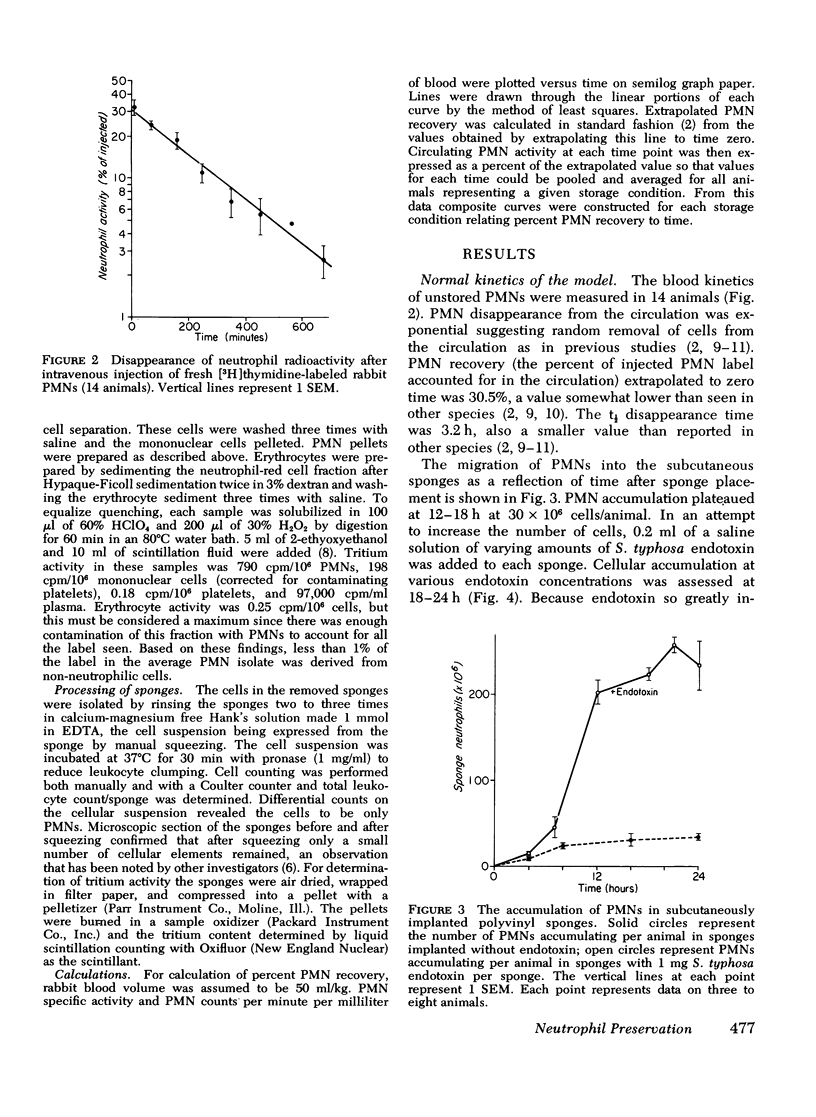
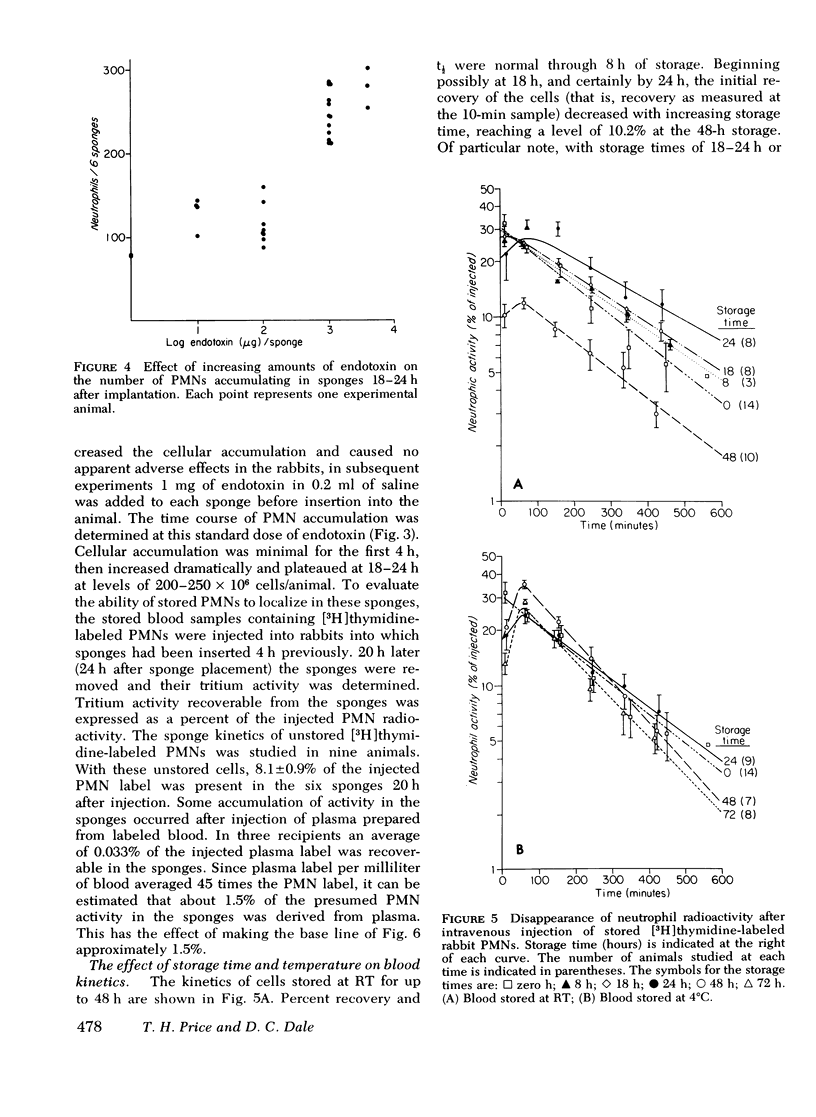
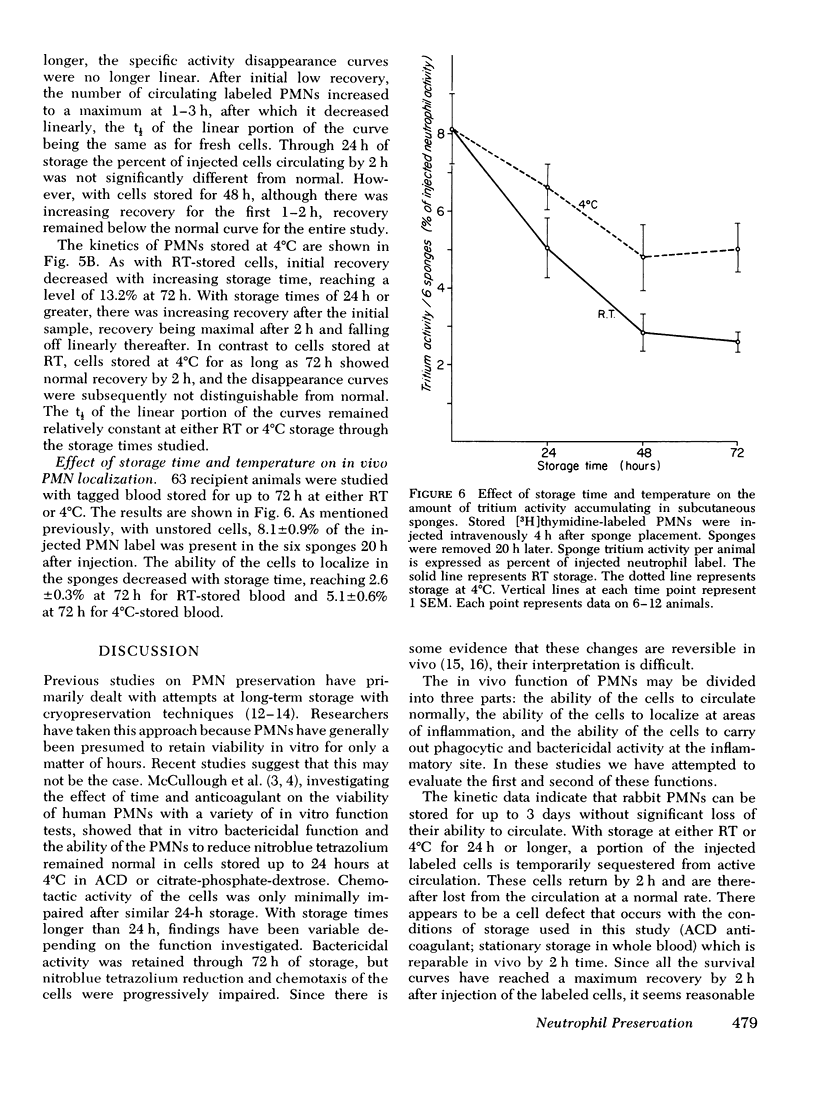
A rabbit model was developed to study the in vivo viability of neutrophils stored in vitro for up to 72 h. Acid-citrate-dextrose anticoagulated whole blood was obtained from rabbits previously injected with tritiated thymidine ([3H]thymidine), stored under varying conditions, and then injected into recipient rabbits. Neutrophil viability and function were assessed by measuring the ability of the tagged neutrophils to circulate and to migrate into subcutaneous polyvinyl sponges. Unstored neutrophils disappeared exponentially from the circulation with a t1/2 of 3.2 h and gave a zero time recovery of 30.5%. Storage of cells at either room temperature or 4 degrees C for 24 h or longer resulted in temporary sequestration of cells from active circulation. With cells stored for up to 72 h at 4 degrees C, recovery returned to normal values after 1-2 h. Room temperature stored cells, in contrast, showed evidence of irreversible damage at 48-h storage with low recovery for the entire time span studied. With unstored blood, 8.1+/-0.9% of the injected neutrophil label was present in the subcutaneous sponges. The accumulated label progressively decreased as cell storage time increased reaching at 72 h 5.1+/-0.6 and 2.6+/-0.3% for 4 degrees C and room temperature-stored cells, respectively. The results of this study indicate that 4 degrees C storage of rabbit neutrophils is superior to storage at room temperature. The data suggest that it may be feasible to store neutrophils at least a few days without loss of in vivo functions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATHENS J. W., RAAB S. O., HAAB O. P., MAUER A. M., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. Leukokinetic studies. III. The distribution of granulocytes in the blood of normal subjects. J Clin Invest. 1961 Jan;40:159–164. doi: 10.1172/JCI104230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavins J. A., Djerassi I., Aghai E., Roy A. J. Current methods for the cryopreservation of human leukocytes (granulocytes). Cryobiology. 1968 Jul-Aug;5(1):60–69. doi: 10.1016/s0011-2240(68)80145-2. [DOI] [PubMed] [Google Scholar]
- Crowley J. P., Rene A., Valeri C. R. The recovery, structure, and function of human blood leukocytes after freeze-preservation. Cryobiology. 1974 Oct;11(5):395–409. doi: 10.1016/0011-2240(74)90106-0. [DOI] [PubMed] [Google Scholar]
- Debelak K. M., Epstein R. B., Andersen B. R. Granulocyte transfusions in leukopenic dogs: in vivo and in vitro function of granulocytes obtained by continuous-flow filtration leukopheresis. Blood. 1974 May;43(5):757–766. [PubMed] [Google Scholar]
- Deubelbeiss K. A., Dancey J. T., Harker L. A., Finch C. A. Neutrophil kinetics in the dog. J Clin Invest. 1975 Apr;55(4):833–839. doi: 10.1172/JCI107994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecke D., Schultze B., Maurer W. Kinetics of neutrophilic granulocytes in the blood of rats. Cell Tissue Kinet. 1973 Jul;6(4):369–378. doi: 10.1111/j.1365-2184.1973.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Malinin T. I. Injury of human polymorphonuclear granulocytes frozen in the presence of cryoprotective agents. Cryobiology. 1972 Apr;9(2):123–130. doi: 10.1016/0011-2240(72)90019-3. [DOI] [PubMed] [Google Scholar]
- Mauer A. M., Athens J. W., Ashenbrucker H., Cartwright G. E., Wintrobe M. M. LEUKOKINETIC STUDIES. II. A METHOD FOR LABELING GRANULOCYTES IN VITRO WITH RADIOACTIVE DIISOPROPYLFLUOROPHOSPHATE (DFP). J Clin Invest. 1960 Sep;39(9):1481–1486. doi: 10.1172/JCI104167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough J., Carter S. J., Quie P. G. Effects of anticoagulants and storage on granulocyte function in bank blood. Blood. 1974 Feb;43(2):207–217. [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- RAAB S. O., ATHENS J. W., HAAB O. P., BOGGS D. R., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. GRANULOKINETICS IN NORMAL DOGS. Am J Physiol. 1964 Jan;206:83–88. doi: 10.1152/ajplegacy.1964.206.1.83. [DOI] [PubMed] [Google Scholar]
- Sanel F. T., Aisner J., Schiffer C. A. Letter: Filtration leukopheresis. Blood. 1976 May;47(5):877–879. [PubMed] [Google Scholar]
- Vincent P. C., Chanana A. D., Cronkite E. P., Joel D. D. The intravascular survival of neutrophils labeled in vivo. Blood. 1974 Mar;43(3):371–377. [PubMed] [Google Scholar]
- Wiener S. L., Wiener R., Urivetzky M., Shafer S., Isenberg H. D., Janov C., Meilman E. The mechanism of action of a single dose of methylprednisolone on acute inflammation in vivo. J Clin Invest. 1975 Sep;56(3):679–689. doi: 10.1172/JCI108138. [DOI] [PMC free article] [PubMed] [Google Scholar]


