Abstract
Rationale
Endothelial cells (EC) at regions exposed to disturbed flow (d-flow) are predisposed to inflammation and the subsequent development of atherosclerosis. We previously showed that thioredoxin interacting protein (TXNIP) was required for tumor necrosis factor (TNF)-mediated expression of vascular cell adhesion molecule (VCAM)-1.
Objective
We sought to investigate the role of TXNIP in d-flow-induced cell adhesion molecule expression and leukocyte interaction with vessels, and the mechanisms by which TXNIP suppresses athero-protective gene expression.
Methods and Results
Using en face staining of mouse aorta, we found a dramatic increase of TXNIP in EC at sites exposed to d-flow as compared to steady flow (s-flow). EC-specific TXNIP knockout (ECTXNIP KO) mice showed significant decreases in VCAM-1 and intercellular adhesion molecule-1 (ICAM-1) mRNA expression in the d-flow regions of mouse aorta. Intravital microscopy of mesenteric venules showed that leukocyte rolling time was decreased, while rolling velocity was increased significantly in EC-TXNIP KO mice. In vitro experiments utilizing a cutout flow chamber to generate varying flow patterns showed that increased TXNIP was required for d-flow-induced EC-monocyte adhesion. Furthermore, we found that the expression of Kruppel-like factor 2 (KLF2), a key anti-inflammatory transcription factor in EC, was inhibited by TXNIP. Luciferase and chromatin immunoprecipitation (ChIP) assays showed that TXNIP was present within a repressing complex on the KLF2 promoter.
Conclusions
These data demonstrate the essential role for TXNIP in mediating EC-leukocyte adhesion under d-flow, as well as define a novel mechanism by which TXNIP acts as a transcriptional corepressor to regulate KLF2-dependent gene expression.
Keywords: TXNIP, KLF2, disturbed flow, cell adhesion molecules
Atherosclerotic lesions occur in a nonrandom pattern characterized by preferential initiation at sites of disturbed blood flow (d-flow) as compared to regions of steady flow (s-flow). Specifically, atherosclerosis is prominent in d-flow regions such as the lesser curvature of the aortic arch, flow dividers, and branch points.1-3 Fluid shear stress, the frictional force generated by blood flow, mechanically acts on endothelial cells (EC) in the vasculature and regulates EC functions. Physiologic s-flow present within straight segments of the arterial tree, is of high shear stress (>10 dyn/cm2) and athero-protective.4, 5 In contrast, d-flow, characterized by a non-unidirectional, non-steady flow pattern with a low magnitude of shear stress (<5dyn/cm2),4, 6 promotes endothelial dysfunction by increasing intracellular reactive oxygen species (ROS) production,7 endothelial permeability,8 and the expression of cell adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1).9 The induction of these cell adhesion molecules in response to d-flow leads to increased binding of circulating leukocytes, which transmigrate across the EC, create chronic inflammation and promote atherogenesis.10
The mechanisms by which s-flow increases athero-protective gene expression have been shown to be mediated primarily (proximately 70%) by a MEK5-ERK5-KLF2 pathway.11 Kruppel-like factor 2 (KLF2) is a key anti-inflammatory transcription factor in EC,11, 12 and is exclusively induced by s-flow but not by d-flow.13 Specifically, KLF2 has been shown to enhance the expression of anti-inflammatory genes such as endothelial nitric oxide synthase (eNOS) on one hand, and inhibit the induction of cell adhesion molecules by cytokines on the other.12
In contrast, less is known regarding specific d-flow mechanisms. Our lab and others have shown that d-flow is associated with increased PKCζ activity14 and p53 activation.15 Previously, we reported that EC thioredoxin interacting protein (TXNIP, also termed as TBP-2) was reduced by s-flow, thereby increasing thioredoxin (Trx) activity and suppressing EC inflammation in response to TNFα via inhibiting JNK and p38 signaling pathways.16 TXNIP has multiple functions in EC including metabolism,17 cell growth,18 and inflammation.19 TXNIP is a member of α-arrestin family20 and acts as a scaffold for several proteins besides Trx,21 including NLRP319 and histone deacetylase (HDAC).18, 22 However, the role of TXNIP in d-flow-induced EC dysfunction has not been investigated. In the present study, we show that the increase of TXNIP is required for d-flow-induced cell adhesion molecule expression and leukocyte adhesion. Furthermore, we show that TXNIP promotes EC dysfunction by acting as a transcriptional corepressor of the KLF2 promoter to inhibit KLF2 expression and its downstream signaling.
Methods
Animals
All animal experiments were conducted in accordance with experimental protocols that were approved by the University Committee on Animal Resources of the University of Rochester. To generate EC-specific TXNIP knockout mice, female TXNIPflox/flox mice (made by Dr Roger Davis, San Diego State University) were crossed with male Cre recombinase transgenic mice under the control of endothelial-specific receptor tyrosine kinase 2 (Tie2) promoter (Tie2-Cre+, purchased from Jackson Lab, #4128) to generate heterozygous Tie2-Cre+ TXNIPflox/-mice for breeding. Then homozygous EC-specific TXNIP knockout mice (Tie2-Cre+TXNIPflox/flox) were obtained and the Cre negative littermates (Tie2-Cre-TXNIPflox/flox) were used as controls. Animals were maintained under pathogen-free conditions of the Aab Cardiovascular Research Institute of the University of Rochester.
Cell culture
HUVEC were cultured as detailed in the Supplemental Material.
Plasmid and siRNA oligonucleotide transfection
HUVEC were transfected with TXNIP-green fluorescent protein (GFP), TXNIP specific siRNA, or luciferase reporter gene constructs as detailed in the Supplemental Material.
Flow Apparatus
Confluent HUVEC cultured in 35-mm dishes were exposed to s- and d-flow at 37°C in a humidified 5% CO2 tissue culture incubator using a parallel-plate flow chamber (GlycoTech, Gaithersburg, MD). Cutouts (1mm×1mm) were made on both sides of the silastic gasket to create shear gradients and vortices within the flow path.23 When culture medium was circulated through the chamber using a peristaltic pump (EP-1 Econo Pump, Bio-Rad, Hercules CA), cells in the main, central part of the flow chamber were exposed to s-flow (12 dyn/cm2) while small disturbed flow areas were created within the cutouts. Shear stress in the s-flow region was calculated by the formula 6μQ/wh2, where Q is the flow rate, μ is the fluid viscosity, w is the width of the channel, and h is the thickness of the gasket. After 24 h, EC in the cutout area exhibited polygonal cell shapes, whereas cells in the middle part of chamber were aligned parallel to the direction of flow. To expose a large amount of cells to flow for biochemical studies, we used a cone and plate flow apparatus as described previously.16, 24 Confluent HUVEC cultured in 100-mm dishes were exposed to s-flow at 12 dyn/cm2 by using smooth cones,16 or exposed to d-flow by using grooved cones at 5 rpm (gives 0.6 dyn/cm2 of s-flow) for the indicated times.16, 24 The shear stress imposed on the surface of the plate was calculated by the formula ωμ/θ where ω is the rotation speed, μ is the fluid viscosity, and θ is the angle of the cone.
Luciferase assay
The luciferase activities of the KLF2 promoter and NF-κB binding site were assayed as detailed in the Supplemental Material.
En face immunofluorescence staining
En face immunofluorescence staining of mouse aorta to compare TXNIP expression in different regions(s-flow vs. d-flow) was performed as detailed in the Supplemental Material.
In vitro THP-1 monocyte adhesion assay
EC-monocyte adhesion was assayed by co-incubating fluorescently labeled THP-1 monocytes with HUVEC as detailed in the Supplemental Material.
Intravital Microscopy
Age-matched male EC-TXNIP knockout and control mice (3-5 months) were anesthetized by intraperitoneal injection of ketamine/xylazine cocktail (0.13/0.0088 mg/g body weight). Leukocytes were labeled by retro-orbital injection of 50 μl 0.05% Rhodamine 6G. Then mesenteric venules were exteriorized and rolling leukocytes were monitored by inverted fluorescent microscope (Nikon Eclipse Ti, Nikon) equipped with a stage warmer (Thermo Plate, Tokai Hit), a QuantEM:512SC camera (Photometrics, Tucson, AZ), and a Nikon S Plan Fluor ELWD 20× lens. Image-Pro V6.2 was used to automatically track moving leukocytes. Then rolling time and velocity were calculated.
Real-time quantitative polymerase chain reaction (PCR)
Relative mRNA expression of VCAM-1, ICAM-1, and KLF2 was quantified by real-time PCR as detailed in the Supplemental Material.
Western blot analysis
Protein expression of TXNIP, VCAM-1, ICAM-1, KLF2, and eNOS was quantified by Western blot as detailed in the Supplemental Material.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay to investigate the association of TXNIP with the KLF2 promoter was performed as detailed in the Supplemental Material.
Statistical analysis
Data are shown as mean ± SD from 3 to 7 independent experiments. Leukocyte rolling events were compared by using Mann-Whitney U test. Other differences were analyzed by two-tailed Student's t test. P values <0.05 were considered statistically significant.
Results
Disturbed flow increases TXNIP expression in EC
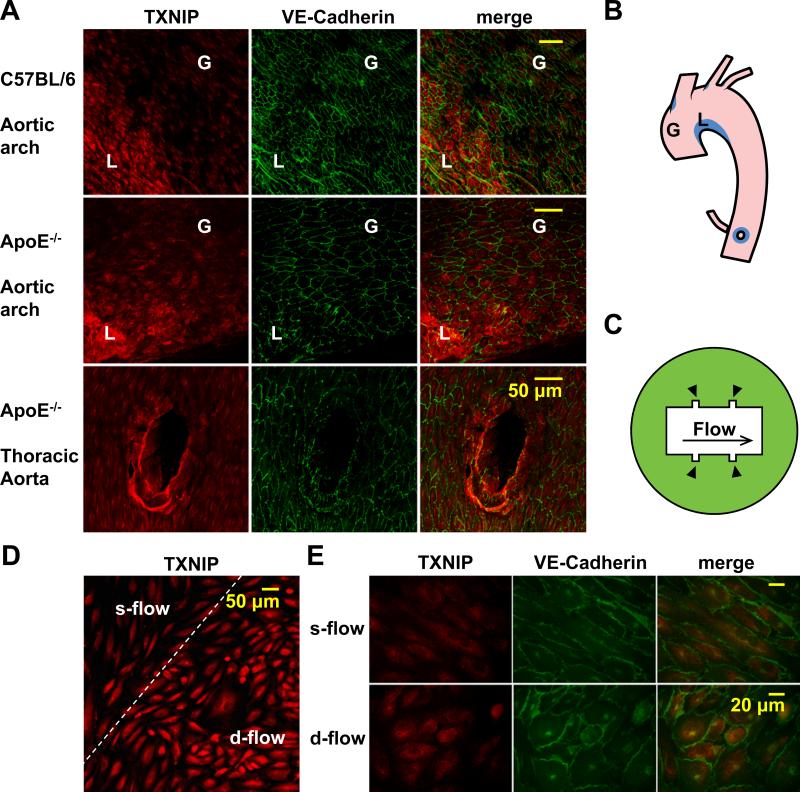
To study the effect of d-flow on TXNIP expression, we performed en face immunofluorescence staining on mouse aorta to compare EC TXNIP expression levels under different flow patterns. We found TXNIP was dramatically increased in d-flow regions, including the lesser curvature of the aortic arch (area ‘L’ in Fig. 1A-B) and the branch points of outflow tracts (bottom panel in Fig. 1A, Online Fig. I, and the corresponding blue area in Fig. 1B), when compared with nearby athero-protective s-flow regions, such as the greater curvature (area ‘G’ in Fig. 1A-B) and the main segment of the thoracic aorta (bottom panel in Fig. 1A) both from C57BL/6 wild type and ApoE-/- mice. To confirm the increase of TXNIP expression by d-flow in vitro, we used a parallel flow chamber with cutouts (Fig. 1C) that creates adjacently located d- and s-flow areas. After 24 h of flow treatment (12 dyn/cm2 in the s-flow region), a greater expression of TXNIP in HUVEC was observed in the d-flow cutout area than in the s-flow region (Fig. 1D-E). Subcellular fractionation further showed that the increase of TXNIP in response to d-flow mainly occurred in the nucleus (Online Fig. II). These results suggest that TXNIP is a mechano-sensitive gene that is highly increased in EC by d-flow both in vivo and in vitro.
Figure 1. Disturbed flow increases TXNIP expression in EC.
(A) Aortas from 12-week-old C57BL/6 (top panel) and ApoE-/- (bottom 2 panels) mice were harvested for en face immunofluorescence analysis in the aortic arch (top and middle panels) and the thoracic aorta (bottom panel) to show TXNIP expression under varying flow patterns from different regions. EC morphology is shown by VE-Cadherin staining. Merge shows increased TXNIP expression in regions of d-flow as defined in Fig. 1B. These regions are demonstrated schematically in an opened mouse aorta (B). Region ‘G’ indicates the greater curvature that is under s-flow. Region ‘L’ indicates the lesser curvature that is under d-flow. D-flow areas are labeled in blue color. (C) A schematic representation of the flow chamber. Arrowheads point to the cutout regions where HUVEC are under d-flow. (D and E) HUVEC cultured in the flow chamber were exposed for 24 h to flow that was calculated to yield shear stress of 12 dyn/cm2 in the s-flow region. TXNIP expression was assayed by immunofluorescence. EC morphology is shown by VE-Cadherin staining.
TXNIP is required for d-flow-induced cell adhesion molecule expression and EC-monocyte adhesion
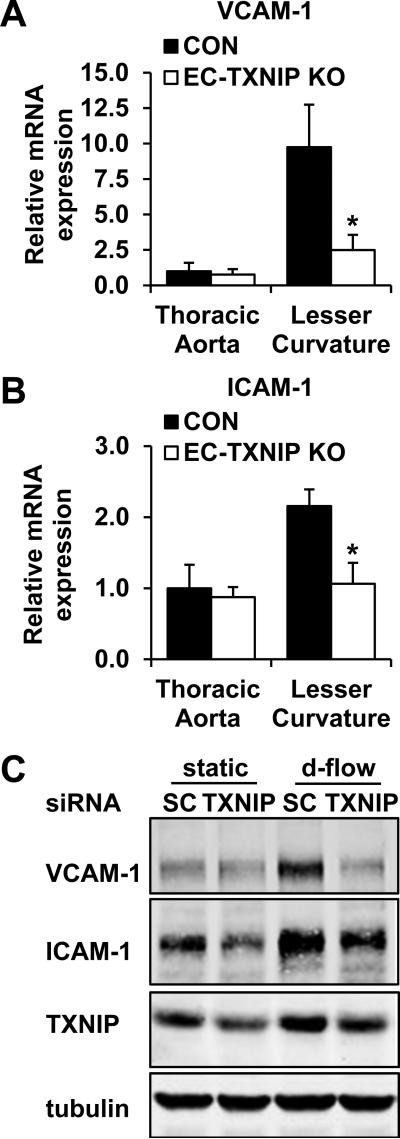
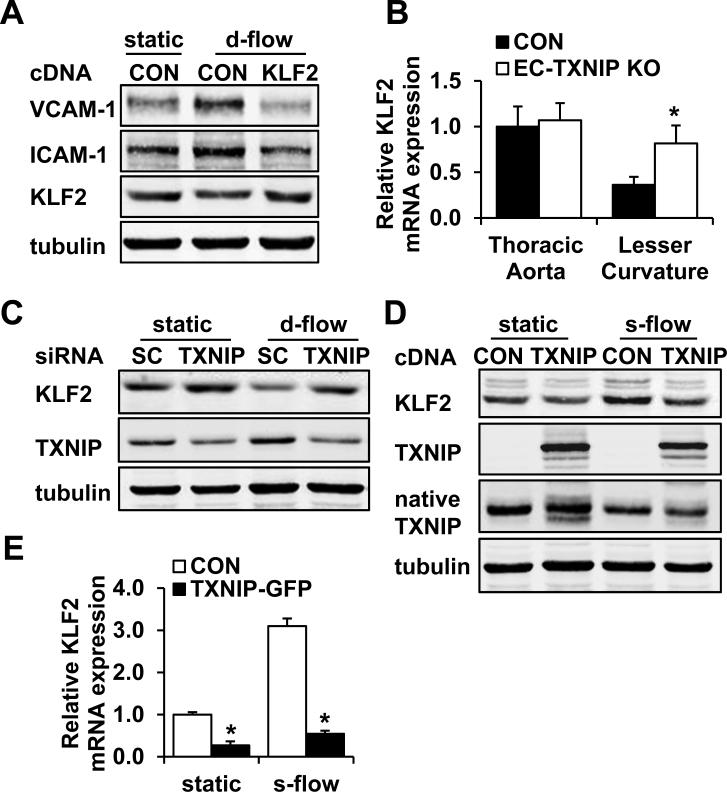
A key reason that d-flow promotes atherosclerosis is the pronounced expression of cell adhesion molecules on the endothelial surface (Online Fig. III)25, 26 which facilitates leukocyte recruitment and vascular inflammation. To investigate whether TXNIP plays a role in this process, we established EC-specific TXNIP knockout (EC-TXNIP KO) mice by using Cre/LoxP strategy. We generated Tie2-Cre+ TXNIPflox/flox mice as knockout mice and Tie2-Cre- TXNIPflox/flox littermates as controls (Online Fig. IV). Then real-time PCR was performed to determine if TXNIP regulates cell adhesion molecule expression. We found that in control animals, VCAM-1 (9.75±2.99-fold) and ICAM-1 (2.15±0.23-fold) mRNA expression levels were markedly increased in the lesser curvature of the aortic arch where the flow pattern is disturbed, as compared to the thoracic aorta where the majority of EC are under s-flow (Fig. 2A-B). Importantly, the increased expression of these cell adhesion molecules in the d-flow area was significantly inhibited in EC-TXNIP KO mice (Fig. 2A-B). In vitro knockdown of TXNIP also showed dramatic decreases in VCAM-1 and ICAM-1 expression under d-flow (Fig. 2C).
Figure 2. TXNIP is required for EC adhesion molecule expression in response to d-flow.
To demonstrate the requirement of TXNIP for d-flow-mediated adhesion molecule expression, the thoracic aorta (s-flow region) and the lesser curvature of the aortic arch (d-flow region) from EC-TXNIP KO mice and control littermates were harvest for RNA extraction. mRNA expression of VCAM-1 (A) and ICAM-1 (B) was detected by quantitative real-time PCR. Data are expressed as mean ± SD from 7 mice in each group. *P<0.05. (C) HUVEC were transfected with either scramble control or TXNIP-specific siRNA for 48 h. Then EC were exposed to d-flow or kept at static condition for an additional 24 h. Protein expression of VCAM-1, ICAM-1 and TXNIP was analyzed by Western blot.
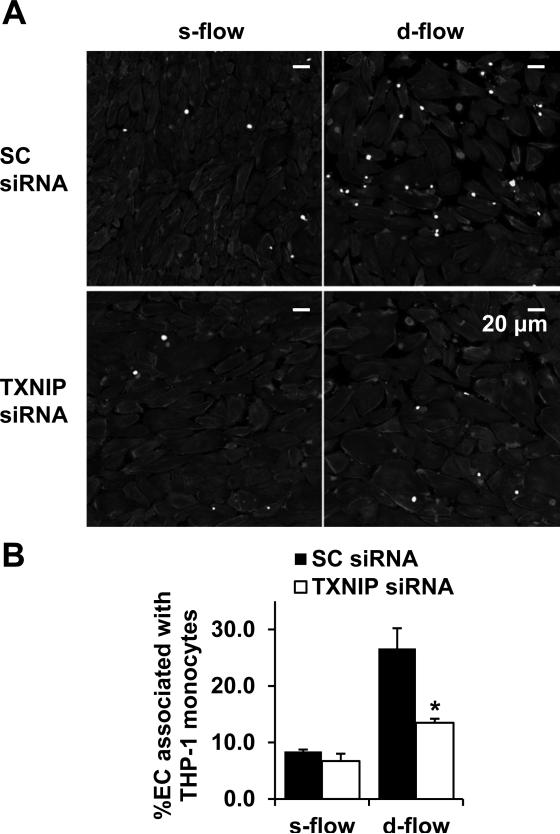
Next, we performed an in vitro adhesion assay to show that TXNIP plays a role in the enhanced EC-leukocyte adhesion under d-flow. HUVEC cultured in the cutout chamber were exposed to flow for 24 h and then co-incubated with pre-labeled THP-1 monocytes. In the control group transfected with scramble control siRNA, there was a >3-fold increase in adherent monocytes in the d-flow cutout area as compared to the adjacent s-flow region (Fig. 3). Importantly, EC-monocyte interactions in response to d-flow were significantly reduced after TXNIP knockdown (Fig. 3). In contrast, the inhibition of EC-monocyte binding by s-flow was virtually abolished in the presence of TXNIP overexpression (Online Fig. V).
Figure 3. TXNIP is required for d-flow-induced EC-THP-1 monocyte adhesion.
(A) HUVEC cultured in the cutout chamber were pretreated with either scramble control or TXNIP-specific siRNA for 48 h, exposed to flow (shear stress = 12 dyn/cm2 in the s-flow region) for an additional 24 h, and then co-incubated with THP-1 monocytes. Shown are adherent monocytes labeled by BCECF-AM. EC morphology is shown by phalloidin staining. (B) Quantification data showing the percentage of EC associated with monocytes in the s- and d-flow area, respectively. Results are expressed as mean ± SD of 3 independent experiments. *P<0.05.
TXNIP deficiency in EC reduces leukocyte-endothelium adhesiveness
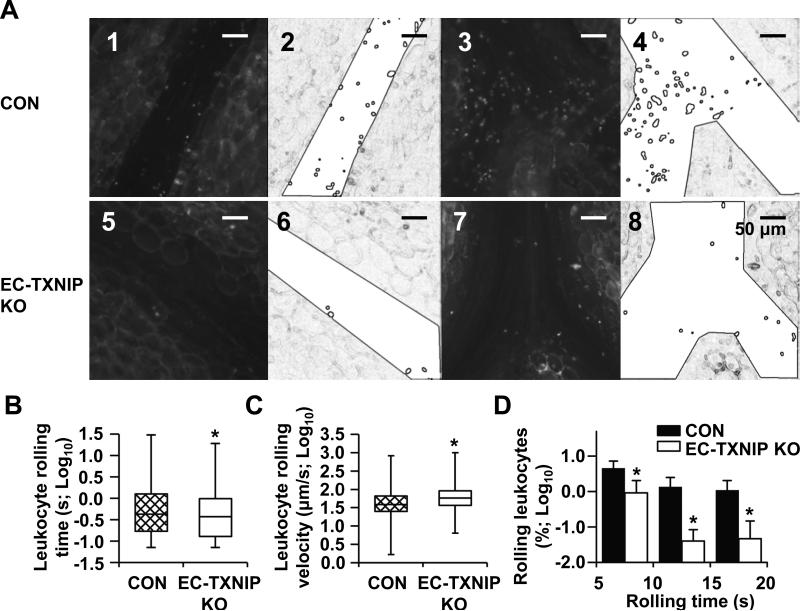
Leukocyte adhesion to endothelium is one of the earliest steps in atherogenesis, which involves the initial attachment of circulating leukocytes, capture, and subsequent firm adherence. Similar as the d-flow area in the conduit artery, the venous system is exposed to low shear stress ranging from 1 to 6 dyn/cm2.4 Moreover, intravital microscopy of mesenteric venules is an established method to analyze leukocyte-endothelium interactions. Thus, to study the role of TXNIP in leukocyte adhesion in vivo, we performed intravital microscopy to quantify leukocyte rolling time and rolling velocity in mesenteric venules (n=7) from control (Tie2-Cre- TXNIPflox/flox) and EC-TXNIP KO (Tie2-Cre+ TXNIPflox/flox) mice. Leukocyte movement was monitored in real time (Fig. 4A). Compared with control animals, significant decreases in rolling time (Fig. 4B) and marked increases in rolling velocity (Fig. 4C) of fluorescently labeled leukocytes were observed in EC-TXNIP KO mice. Especially, the number of leukocytes with long rolling time (5 s-20 s) was substantially decreased in EC-TXNIP KO mice (Fig. 4D). In addition, no significant difference in EC-leukocyte adhesion was detected between Tie2-Cre- TXNIPflox/flox and Tie2-Cre+ transgenic mice (data not shown), which confirms that the decrease of endothelial adhesiveness in EC-TXNIP KO mice is due to the depletion of TXNIP but not Cre toxicity. Comparison of the two strains showed no changes in systolic blood pressure (Online Fig. VIA), heart rate (Online Fig. VIB), and TXNIP expression in peripheral blood mononuclear cells (PBMC; Online Fig. VII). Taken together, these results clearly show that TXNIP deficiency reduces leukocyte adhesion to endothelium, which confirms the role of TXNIP in modulating the expression of pro-inflammatory cell adhesion molecules.
Figure 4. TXNIP deficiency in EC reduces leukocyte-endothelium adhesiveness.
(A) Representative images of rolling leukocytes within mesenteric venules from EC-TXNIP KO mice (panels 5 and 7) and control littermates (panels 1 and 3). The recorded videos were processed by Image-Pro into a simplified form for the automatic recognition and tracking of rolling leukocytes. The corresponding processed images are also shown (panels 6 and 8, and panels 2 and 4). (B and C) Whisker plots showing the leukocyte rolling time (B) and rolling velocity (C) from both animal groups after log transformation. Median, quartiles and extreme values are given. Significant differences between two animal groups were tested using Mann-Whitney U test. (D) Further analysis of the quantified data for slow moving leukocytes with rolling time >5 s. Percentage of leukocytes with indicated rolling times was calculated correspondingly by using frequency table. Data are expressed as mean ± SD from 7 mice in each group. *P<0.05.
TXNIP suppresses KLF2 expression and promoter activity
KLF2 has been reported to be an important anti-inflammatory transcription factor in EC.12 It is highly induced by s-flow but not by d-flow,13, 27 and antagonizes the induction of cell adhesion molecules in response to TNFα and IL-1β in EC.12 Thus, we next hypothesized that KLF2 is regulated by TXNIP in EC in a flow dependent manner. We found KLF2 expression was decreased by d-flow (Fig. 5A). Moreover, overexpression of KLF2 virtually abolished the d-flow-induced VCAM-1 and ICAM-1 expression (Fig. 5A), suggesting that the inhibition of KLF2 by d-flow is required for cell adhesion molecule expression.
Figure 5. TXNIP regulates cell adhesion molecule expression by repressing KLF2.
(A) To demonstrate that the repression of KLF2 by d-flow leads to cell adhesion molecule expression, HUVEC in 100-mm dish were transfected with 3 μg KLF2 cDNA or control plasmid , and exposed to d-flow or kept at static condition for 24 h. VCAM-1, ICAM-1 and KLF2 expression levels were analyzed by Western blot. (B) To demonstrate the role of TXNIP in d-flow-induced KLF2 inhibition, mRNA expression of KLF2 in the thoracic aorta (s-flow region) and the lesser curvature of the aortic arch (d-flow region) from EC-TXNIP KO mice and control littermates was analyzed by quantitative real-time PCR. Data are expressed as mean ± SD from 7 mice in each group. *P<0.05. (C) HUVEC were transfected with either scramble control or TXNIP-specific siRNA for 48 h. Then EC were exposed to d-flow or kept at static condition for an additional 24 h. Protein expression of KLF2 and TXNIP was analyzed by Western blot. (D and E) To demonstrate that TXNIP inhibits KLF2 expression, HUVEC in 100-mm dish were transfected with 3 μg TXNIP-GFP or control plasmid, and exposed to s-flow or kept at static condition for 24 h for protein harvest (D) or 6 h for RNA harvest (E). (D) KLF2 protein expression levels were then analyzed by Western blot. (E) KLF2 mRNA levels were quantified by real-time PCR and are expressed as mean ± SD of 3 independent experiments. *P<0.05.
Next, we found that KLF2 mRNA levels in the lesser curvature of the aortic arch (d-flow region) were significantly increased in EC-TXNIP KO mice by using real-time PCR (Fig. 5B). Furthermore, knockdown of TXNIP in vitro increased KLF2 protein levels under both static condition and d-flow (Fig. 5C). KLF2 has been previously reported to prevent NF-κB activation.27 We also found that the d-flow-induced NF-κB activation was completely abolished after TXNIP knockdown (Online Fig. VIII). On the other hand, overexpression of TXNIP significantly reduced KLF2 protein (Fig. 5D) and mRNA (Fig. 5E) expression levels under both static condition and s-flow. In addition, the expression of eNOS, a downstream target of KLF2 in EC, was also decreased by TXNIP overexpression (Online Fig. IXA).
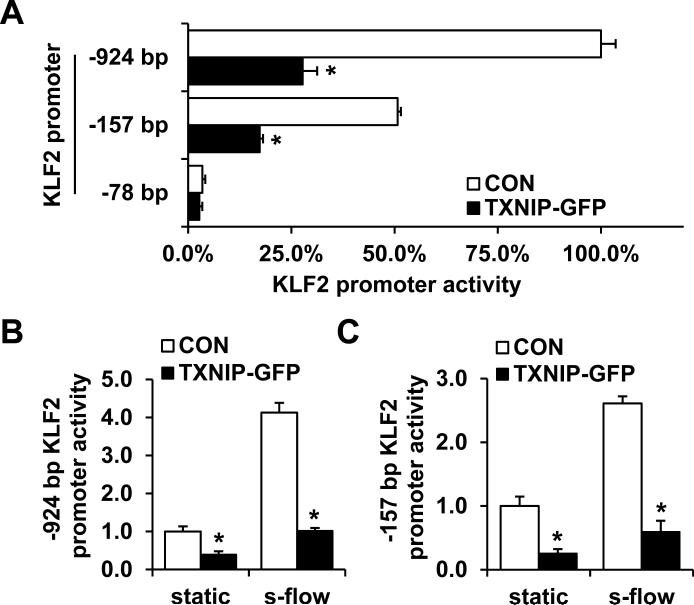
To establish the mechanism by which TXNIP inhibits KLF2 expression, we studied the effect of TXNIP on KLF2 promoter activity by using luciferase assay. TXNIP overexpression significantly decreased the activity of both the -924 bp full-length promoter and the -157 bp fragment, whereas a further deletion of the promoter (-78 bp construct) resulted in complete loss of inhibition (Fig. 6A). These data suggest that the TXNIP-mediated repression occurs through a region from -157 bp to -78 bp of the KLF2 promoter. The -157 bp promoter fragment was previously reported to be responsible for the transactivation of KLF2 by s-flow.28 Thus, we further investigated whether the inhibition persisted after s-flow exposure. We detected a 4.13±0.13-fold increase for the -924 bp promoter construct (Fig. 6B) and a 2.61±0.11-fold increase for the -157 bp promoter construct (Fig. 6C) in luciferase activity after s-flow treatment, whereas the increase of promoter activity by s-flow was significantly repressed in the presence of TXNIP overexpression for both promoters. Taken together, these results suggest a role for TXNIP as a corepressor of the KLF2 promoter, thereby inhibiting KLF2 expression and its downstream signaling. These data are consistent with the reports that TXNIP associates with transcriptional repressors such as HDAC1 and HDAC3.18, 22
Figure 6. TXNIP suppresses KLF2 promoter activity.
(A) Various deletion constructs (-924 bp, -157 bp and -78 bp) of the KLF2 promoter were co-transfected with TXNIP-GFP or control plasmid into HUVEC. After 24 h of transfection, cells were harvested and the luciferase activities were measured. (B and C) HUVEC were co-transfected with either TXNIP-GFP or control plasmid, and -924 bp (B) or -157 bp (C) KLF2 promoter reporter gene construct. After 18 h of incubation, cells were exposed to s-flow or kept at static condition for 6 h before harvest for luciferase assay. Results are expressed as mean ± SD of 3 independent experiments. *P<0.05.
Association of TXNIP with the KLF2 promoter is increased by d-flow and reduced by s-flow
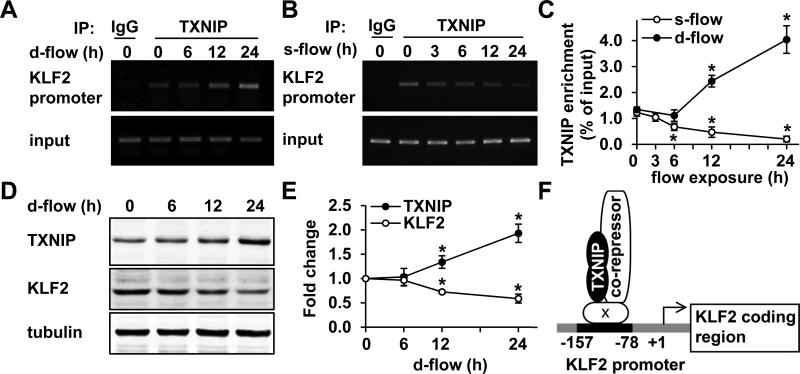
Based on our luciferase assay results, we proposed that TXNIP is a transcriptional corepressor of the KLF2 promoter. To test this, chromatin immunoprecipitation (ChIP) assays were performed to analyze the association of TXNIP with the KLF2 promoter under different flow conditions. There was a significant increase in TXNIP-KLF2 promoter complex when cells were exposed to d-flow (peak increase of 4.04±0.53-fold at 24 h, Fig. 7A, C). In contrast, this complex was decreased by s-flow exposure (peak decrease of 0.20±0.11-fold at 24 h, Fig. 7B, C). Next, we showed that d-flow treatment altered protein expression in a manner consistent with the ChIP results: increased TXNIP by 1.93±0.19-fold and decreased KLF2 by 0.59±0.09-fold at 24 h (Fig. 7D-E). A mild decrease in eNOS expression after d-flow exposure was also observed (Fig. IXB). Taken together, these results indicate that TXNIP is a member of the transcriptional repressing complex localized on the KLF2 promoter (Fig. 7F). This complex is increased by d-flow and decreased by s-flow, which finally leads to the deactivation or activation of KLF2 transcription and its downstream signaling.
Figure 7. Association of TXNIP with the KLF2 promoter is increased by d-flow and reduced by s-flow.
ChIP assays were performed to analyze the enrichment of TXNIP on the KLF2 promoter at the indicated times after d-flow (A, C) or s-flow (B, C) exposure. (A-B) Shown are PCR produces amplified from input or immunoprecipitated DNA with TXNIP antibody or control IgG. (C) Quantifications of the ChIP experiments were performed by using real-time PCR. The percentage of immunoprecipitated DNA relative to the input control is shown. Results are expressed as mean ± SD of 3 independent experiments. *P<0.05. (D-E) HUVEC were exposed to d-flow for the indicated times. TXNIP and KLF2 expression was analyzed by Western blot (D) and the densitometric quantifications are shown (E). Results are expressed as mean ± SD of 3 independent experiments. *P<0.05. (F) A schematic representation showing the mechanism by which TXNIP regulates KLF2 promoter activity. Letter ‘x’ indicates an unknown factor.
Discussion
The major findings of the present study are that TXNIP expression is increased by d-flow, inhibits KLF2 expression and promotes endothelial-leukocyte adhesion; thereby initiating inflammation (Online Fig. X). The ChIP assay data show the mechanism for TXNIP regulation of KLF2 involves the association with the KLF2 promoter. In addition, an important feature of the current study is that we clearly demonstrate by 2 approaches that d-flow athero-prone sites exhibit a dramatic increase of TXNIP expression. First, en face immunohistochemistry shows high TXNIP expression in d-flow areas, which is very similar to PKCζ as we previously described.14 Second, in vitro studies of s-flow and d-flow using a cutout chamber under identical tissue culture conditions reproduce the in vivo results.23 These results lead to the concept that TXNIP is a mechanosensitive switch for EC inflammation by inhibiting athero-protective pathways downstream of KLF2, and activating athero-promoting gene expression.
We propose that TXNIP is a very important regulator of the genetic programs present under s-flow and d-flow by regulation of KLF2. It is likely that s-flow inhibits TXNIP interaction with the KLF2 promoter by two mechanisms. First, total TXNIP expression in EC is decreased by s-flow in a chronic manner.16 Second, s-flow may maintain TXNIP in a cytosolic location that prevents effects on gene transcription. In contrast, d-flow dramatically increases TXNIP expression in the nucleus, which may lead to increased interactions with transcriptional repressors, such as HDAC1 and HDAC3.18, 22 Thus, TXNIP seems to act as a corepressor by recruiting transcriptional repressors to the transcription factors that are responsible for KLF2 transactivation (Fig. 7F). Furthermore, we established that the activation of the KLF2 promoter in response to s-flow was virtually abolished by TXNIP overexpression, and a small fragment of the KLF2 promoter (-157 bp to -78 bp) was responsible (Fig. 6A). Analysis of the promoter sequence and a literature survey failed to yield any known binding motif or associated transcription factor. Identification of the precise mechanism of TXNIP corepression will be an important future goal.
While TXNIP appears to be a common mediator for regulation of s-flow and d-flow gene expression, the mechanisms by which flow regulates TXNIP and KLF2 expression clearly differ. KLF2 expression is regulated by several transcription factors, such as MEF2C,29 PLZF,30 and P30031 or by miRNA post-transcriptionally.32 Using in situ hybridization, Dekker et al. showed that KLF2 is exclusively induced by s-flow but not d-flow.13 Our study shows a novel mechanism by which KLF2 is differentially regulated that involves transcription corepression by d-flow. Several mechanisms have been shown to regulate TXNIP expression. To date, these fall into either metabolic or redox-sensitive pathways. For example, the MondoA:Mlx dimer transcription factors promote TXNIP expression by activating a carbohydrate response element (ChoRE) on the TXNIP promoter.33 Among the pathways responsive to oxidative stress, both hyperglycemia and d-flow increase intracellular ROS levels in EC by activating NAD(P)H oxidase,7, 34, 35 raising the possibility that a similar mechanism may be responsible for d-flow-induced TXNIP. In addition, Nrf2, an important shear stress-responsive transcription factor regulating redox-sensitive responses,36 has been shown to significantly increase TXNIP expression.37 The roles of MondoA:Mlx and Nrf2 as mediators for TXNIP induction by d-flow need further elucidation.
Our findings have several potential clinical implications. First, as shown by intravital microscopy (Fig. 4), we demonstrated that TXNIP is required for leukocyte interaction with vessel walls, suggesting it is a novel target for preventing leukocyte recruitment. Second, the increase of TXNIP in response to d-flow promotes cell adhesion molecule expression; and conversely this effect is inhibited by overexpression of KLF2. This establishes a counterregulatory role for TXNIP and KLF2, which suggests that a strategy to increase KLF2 or decrease TXNIP should provide therapeutic benefits. Finally, given that diabetes is associated with high TXNIP expression, therapies that decrease TXNIP would be predicted to be useful for reducing complications in diabetics such as accelerated atherosclerosis.38, 39
Supplementary Material
Novelty and Significance.
What is known?
Thioredoxin interacting protein (TXNIP), a scaffold protein that is a member of the α-arrestin family, is required for TNF-induced endothelial cell (EC) inflammation.
Disturbed flow (d-flow) leads to EC inflammation and the development of atherosclerosis by increasing cell adhesion molecule expression and EC-leukocyte adhesion.
Steady flow (s-flow) decreases EC TXNIP expression.
Kruppel-Like Factor 2 (KLF2), a key anti-inflammatory transcription factor in EC, is highly induced by s-flow, but not by d-flow.
What new information does this article contribute?
Enhanced TXNIP expression is responsible for d-flow-induced cell adhesion molecule expression and EC-leukocyte adhesion.
TXNIP acts as a transcriptional corepressor of the KLF2 promoter, thereby inhibiting KLF2 expression and its downstream signaling.
TXNIP-KLF2 promoter complex is increased by d-flow and decreased by s-flow.
Atherosclerotic lesions preferentially initiate in the d-flow areas of the arterial tree, such as the lesser curvature of the aortic arch and the branch points of outflow tracts. However, the mechanism by which d-flow promotes EC inflammation is not clear. By using en face staining of mouse aorta in vivo and cutout flow chamber in vitro, we showed that TXNIP is a mechanosensitive gene that is dramatically increased by d-flow. Importantly, the increase of TXNIP is required for the enhanced expression of cell adhesion molecules and EC-leukocyte adhesion under d-flow. Specifically, TXNIP promotes EC inflammation by acting as a transcriptional corepressor of the KLF2 promoter, thereby inhibiting KLF2-dependent anti-inflammatory pathways. These findings provide new insight into the role of TXNIP as a mechanosensitive switch for EC inflammation by inhibiting athero-protective pathways downstream of KLF2, and activating athero-promoting gene expression.
Acknowledgments
We thank Craig Morrell for the support of intravital microscopy, Kyung-Sun Heo for the support of en face immunofluorescence staining, and Amy Mohan, Christine Christie and Alison Hobbins for technical assistance.
Sources of Funding
This work was supported by National Institutes of Health Grants HL077789 and HL106158 (to B.C.B. and to C.Y.).
Non-standard Abbreviations and Acronyms
- ChIP
chromatin immunoprecipitation
- ChoRE
carbohydrate response element
- D-FLOW
disturbed flow
- EC
endothelial cell(s)
- EC-TXNIP KO
EC-specific TXNIP knockout
- eNOS
endothelial nitric oxide synthase
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- HDAC
histone deacetylase
- HUVEC
human umbilical vein endothelial cell(s)
- ICAM-1
intercellular adhesion molecule-1
- KLF2
Kruppel-like factor 2
- PBMC
peripheral blood mononuclear cell(s)
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- S-FLOW
steady flow
- TBS
Tris buffered saline
- Tie2
endothelial-specific receptor tyrosine kinase 2
- TNF
tumor necrosis factor
- Trx
thioredoxin
- TXNIP
thioredoxin interacting protein
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Patrizia Nigro is currently at Laboratorio di Biologia Vascolare e Medicina Rigenerativa Centro Cardiologico Monzino-IRCCS, Via Parea 4, 20138 Milano, Italia
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakashima Y, Plump A, Raines E, Breslow J, Ross R. Apoe-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arteriosclerosis, Thrombosis, and Vascular Biology. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 2.Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature. 1969;223:1159–1160. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- 3.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 4.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 5.Cooke JP. Flow, no, and atherogenesis. Proc Natl Acad Sci U S A. 2003;100:768–770. doi: 10.1073/pnas.0430082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 7.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state : Role of a superoxide-producing nadh oxidase. Circulation Research. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 8.Himburg HA, Grzybowski DM, Hazel AL, LaMack JA, Li XM, Friedman MH. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am J Physiol Heart Circ Physiol. 2004;286:H1916–1922. doi: 10.1152/ajpheart.00897.2003. [DOI] [PubMed] [Google Scholar]
- 9.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circulation Research. 1998;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 10.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 11.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA, Jr., Garcia-Cardena G. Integration of flow-dependent endothelial phenotypes by kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr., Garcia-Cardena G, Jain MK. Klf2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung kruppel-like factor (klf2). Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 14.Nigro P, Abe J, Woo CH, Satoh K, McClain C, O'Dell MR, Lee H, Lim JH, Li JD, Heo KS, Fujiwara K, Berk BC. Pkczeta decreases enos protein stability via inhibitory phosphorylation of erk5. Blood. 2010;116:1971–1979. doi: 10.1182/blood-2010-02-269134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heo KS, Lee H, Nigro P, Thomas T, Le NT, Chang E, McClain C, Reinhart-King CA, King MR, Berk BC, Fujiwara K, Woo CH, Abe J. Pkc{zeta} mediates disturbed flow-induced endothelial apoptosis via p53 sumoylation. J Cell Biol. 2011;193:867–884. doi: 10.1083/jcb.201010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest. 2005;115:733–738. doi: 10.1172/JCI200523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oka S, Liu W, Masutani H, Hirata H, Shinkai Y, Yamada S, Yoshida T, Nakamura H, Yodoi J. Impaired fatty acid utilization in thioredoxin binding protein-2 (tbp-2)-deficient mice: A unique animal model of reye syndrome. FASEB J. 2006;20:121–123. doi: 10.1096/fj.05-4439fje. [DOI] [PubMed] [Google Scholar]
- 18.Han SH, Jeon JH, Ju HR, Jung U, Kim KY, Yoo HS, Lee YH, Song KS, Hwang HM, Na YS, Yang Y, Lee KN, Choi I. Vdup1 upregulated by tgf-beta1 and 1,25-dihydorxyvitamin d3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene. 2003;22:4035–4046. doi: 10.1038/sj.onc.1206610. [DOI] [PubMed] [Google Scholar]
- 19.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in nlrp3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez CE. On the origins of arrestin and rhodopsin. BMC Evol Biol. 2008;8:222. doi: 10.1186/1471-2148-8-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. Identification of thioredoxin-binding protein-2/vitamin d(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 22.Kwon HJ, Won YS, Suh HW, Jeon JH, Shao Y, Yoon SR, Chung JW, Kim TD, Kim HM, Nam KH, Yoon WK, Kim DG, Kim JH, Kim YS, Kim DY, Kim HC, Choi I. Vitamin d3 upregulated protein 1 suppresses tnf-alpha-induced nf-kappab activation in hepatocarcinogenesis. J Immunol. 2010;185:3980–3989. doi: 10.4049/jimmunol.1000990. [DOI] [PubMed] [Google Scholar]
- 23.Reinhart-King CA, Fujiwara K, Berk BC. Physiologic stress-mediated signaling in the endothelium. Methods Enzymol. 2008;443:25–44. doi: 10.1016/S0076-6879(08)02002-8. [DOI] [PubMed] [Google Scholar]
- 24.Heo K-S, Lee H, Nigro P, Thomas T, Le N-T, Chang E, McClain C, Reinhart-King CA, King MR, Berk BC, Fujiwara K, Woo C-H, Abe J-i. Pkcζ mediates disturbed flow-induced endothelial apoptosis via p53 sumoylation. The Journal of Cell Biology. 2011;193:867–884. doi: 10.1083/jcb.201010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 26.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen KL, Hamik A, Jain MK, McCrae KR. Endothelial cell activation by antiphospholipid antibodies is modulated by kruppel-like transcription factors. Blood. 2011;117:6383–6391. doi: 10.1182/blood-2010-10-313072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huddleson JP, Srinivasan S, Ahmad N, Lingrel JB. Fluid shear stress induces endothelial klf2 gene expression through a defined promoter region. Biol Chem. 2004;385:723–729. doi: 10.1515/BC.2004.088. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Lin Z, SenBanerjee S, Jain MK. Tumor necrosis factor alpha-mediated reduction of klf2 is due to inhibition of mef2 by nf-kappab and histone deacetylases. Mol Cell Biol. 2005;25:5893–5903. doi: 10.1128/MCB.25.14.5893-5903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huddleson JP, Ahmad N, Srinivasan S, Lingrel JB. Induction of klf2 by fluid shear stress requires a novel promoter element activated by a phosphatidylinositol 3-kinase-dependent chromatin-remodeling pathway. J Biol Chem. 2005;280:23371–23379. doi: 10.1074/jbc.M413839200. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Bacanamwo M, Harrison DG. Activation of p300 histone acetyltransferase activity is an early endothelial response to laminar shear stress and is essential for stimulation of endothelial nitric-oxide synthase mrna transcription. J Biol Chem. 2008;283:16293–16298. doi: 10.1074/jbc.M801803200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr., Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY. Flow-dependent regulation of kruppel-like factor 2 is mediated by microrna-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu FX, Chai TF, He H, Hagen T, Luo Y. Thioredoxin-interacting protein (txnip) gene expression: Sensing oxidative phosphorylation status and glycolytic rate. J Biol Chem. 2010;285:25822–25830. doi: 10.1074/jbc.M110.108290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and nad(p)h oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 35.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase c--dependent activation of nad(p)h oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 36.Chen XL, Dodd G, Thomas S, Zhang X, Wasserman MA, Rovin BH, Kunsch C. Activation of nrf2/are pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am J Physiol Heart Circ Physiol. 2006;290:H1862–1870. doi: 10.1152/ajpheart.00651.2005. [DOI] [PubMed] [Google Scholar]
- 37.Adair-Kirk TL, Atkinson JJ, Griffin GL, Watson MA, Kelley DG, DeMello D, Senior RM, Betsuyaku T. Distal airways in mice exposed to cigarette smoke: Nrf2-regulated genes are increased in clara cells. Am J Respir Cell Mol Biol. 2008;39:400–411. doi: 10.1165/rcmb.2007-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem. 2004;279:30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- 39.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.