Abstract
Bacterial transcription factors often function as DNA-binding proteins that selectively activate or repress promoters, although the biochemical mechanisms vary. In most well-understood examples, activators function by either increasing the affinity of RNA polymerase (RNAP) for the target promoter, or by increasing the isomerization of the initial closed complex to the open complex. We report that Bacillus subtilis Btr, a member of the AraC family of activators, functions principally as a ligand-dependent activator of promoter clearance. In the presence of its co-activator, the siderophore bacillibactin (BB), the Btr:BB complex enhances productive transcription, while having only modest effects on either RNAP promoter association or the production of abortive transcripts. Btr binds to two direct repeat sequences adjacent to the −35 region; recognition of the downstream motif is most important for establishing a productive interaction between the Btr:BB complex and RNAP. The resulting Btr:BB dependent increase in transcription enables the production of the ferric-BB importer to be activated by the presence of its cognate substrate.
INTRODUCTION
Transcription factors determine the global landscape of gene expression. Bacterial regulatory proteins most often act on transcription initiation, but the precise mechanisms vary. Transcription initiation is a complex, multistep process. In general, promoter recognition can be divided into an initial binding event (leading to a transient, closed complex, RPC), isomerization to one or more intermediate (RPi) complexes, and finally formation of the fully strand-separated open complex (RPO) (1). Upon binding of initiating nucleotides, RNA polymerase (RNAP) forms an initial transcribing complex (RPITC) and commences synthesis of short (usually up to ∼10–12 nt) RNA products which may or may not be released in a repetitive reaction termed abortive synthesis (2,3). Promoter escape is thought to be correlated with the loss of σ contacts to the promoter, release of σ from the elongating ternary complex, and conversion of RPITC into the highly stable and processive elongation complex (RPE) (1,4).
Bacterial RNAP is directed to specific target promoters by an associated σ subunit which contacts DNA regions centered near −10 and −35 relative to the transcription start site (5,6). Repressor proteins bound near or overlapping the promoter recognition elements can impede RNAP binding or promoter escape. In many cases, simple occlusion of the promoter suffices for repression (7). In contrast, activation requires activator:RNAP interactions that increase the rate of the slowest (rate-limiting) step in initiation (8).
Mechanisms of activation have been studied, to various levels of refinement, for dozens of different bacterial activators. In most cases, activators bind adjacent to, and upstream of, RNAP and establish protein–protein interactions with either the α-subunit C-terminal domain (αCTD) or region 4 of the σ subunit (9,10). These interactions may serve to increase the affinity of RNAP for the promoter region (initial binding event; KB) or the rate of the subsequent isomerization step(s) (kf) leading to the formation of the transcriptionally competent open complex (8). Historically, activators that affect KB versus kf were often distinguished using abortive initiation assays in which the rate of product synthesis was monitored as a function of RNAP concentration (3). This assay, as developed using Escherichia coli σ70 holoenzyme, allows the early steps in transcription initiation to be separated into those that are dependent on RNAP concentration (KB) and those that are independent (kf). RNAP binding affinity can be independently monitored using direct methods such as the electrophoretic mobility shift assay (EMSA) or DNase I footprinting (11), while DNA isomerization to RPO can be monitored using chemical (KMnO4) sensitivity to monitor DNA melting (12). Depending on the details of the initiation pathway, the rate-limiting step assigned as kf may or may not correspond to DNA melting.
While most activators exert their primary effects on either initial binding or promoter isomerization, at some promoters later stages may be rate-limiting and therefore appropriate targets for activator proteins. For example, transcription activators may stimulate either the initiation or early elongation steps of RNA synthesis (regulators acting after the transition from RPITC to RPE are classified instead as elongation factors; 13). Classic examples of late-acting activator proteins include E. coli CRP at the malT promoter and λ Q at PR′. In the former case, CRP activates the production of full length transcripts while having little effect on either promoter binding or initiation (as measured using an abortive initiation assay) leading to the suggestion that CRP specifically increases the rate of promoter escape (14). Subsequent studies, however, support a model in which CRP acts early in initiation to favor formation of an escape-competent open complex (15,16). The λ Q protein acts on a promoter-proximal, paused elongation complex to stimulate promoter escape (17,18). Promoter escape, the conversion of the RPITC (which can release abortive products and revert to RPO) into the stable and highly processive RPE complex, is generally accompanied by σ release although exceptions may be more prevalent than previously appreciated (19,20).
Recent studies suggest a correlation between the presence of σ factor recognition elements in the early transcribed region and stalling of RPITC (promoter-proximal pausing) (18,21). First recognized in the case of λ phage PR′, recognition of a downstream promoter-like element by the still associated σ subunit leads to pausing of the RPITC which provides a target for Q modification. The Q-modified RNAP efficiently escapes the pause to generate full-length transcripts (17). More generally, σ-dependent pausing during promoter clearance increases the association of σ during elongation and thereby modifies the properties of RPE, likely by competing for binding with elongation factors (20). In Bacillus subtilis, the GreA transcript cleavage factor has been proposed to associate with early paused elongation complexes to stimulate escape of RNAP from the promoter (22). Studies in E. coli suggest that promoter-proximal pausing may affect the transcription of a large fraction (estimated at >20%) of transcription units (20). Evidence for the widespread occurrence, and regulatory impact, of promoter-proximal pausing has also emerged in a variety of model eukaryotes (23,24).
Here, we have investigated the mechanism of transcription activation by B. subtilis Btr (25), an unusual member of the AraC family of transcription factors (26). Biochemical studies of AraC family activators are often challenging, due to the difficulties with both over-expression and purification (27,28). As a result, only a handful of AraC family proteins have been biochemically characterized (28–31). Btr contains an amino-terminal DNA-binding and dimerization domain appended to a carboxy-terminal ligand-binding domain structurally and functionally related to siderophore substrate binding proteins associated with iron import (25). Btr binds upstream of the promoter for the feuABC operon (PfeuA) encoding an ABC transporter for the import of ferric-bacillibactin (BB), a catecholate siderophore made by various Bacillus spp. In the presence of BB (and to a lesser extent, de-ferrated BB), the Btr:BB complex strongly activates transcription from PfeuA. The mechanism of activation is shown here to involve effects on multiple steps, but most prominently a large and ligand-activated increase in the formation of productive transcripts due to an increase in the rate of promoter escape.
MATERIALS AND METHODS
Strain construction and growth conditions
Bacillus subtilis was grown in Luria–Bertani (LB) medium or in a MOPS-based minimal medium, FS-MM (32). Unless otherwise indicated, liquid media were inoculated from an overnight preculture and incubated at 37°C with shaking at 200 rpm. For selection, antibiotics were added at the following concentrations: erythromycin (1 µg/ml) and lincomycin (25 µg/ml) [for selecting for macrolide–lincosamide–streptogramin B (MLS) resistance], spectinomycin (100 µg/ml), chloramphenicol (10 µg/ml), kanamycin (15 µg/ml) and neomycin (10 µg/ml). Routine molecular biology procedures were carried out using E. coli DH5α for DNA cloning as described in Ref. (33). Isolation of B. subtilis chromosomal DNA, transformation and specialized SPβ transduction were performed according to (34). Restriction enzymes, DNA ligase and DNA polymerases were used according to the manufacturer's instructions (New England Biolabs).
To construct an feuA-lacZ transcriptional fusion, the feuA regulatory region (PfeuA) was amplified from genomic DNA by PCR and cloned as a HindIII-BamHI fragment into pJPM122 (35). The resulting construct was linearized with ScaI and used to transform strain ZB307A (36) to neomycin resistance by integration into the temperature sensitive SPβ prophage. An SPβ transducing lysate was prepared by heat induction (35) and used to transduce the resulting PfeuA-lacZ transcriptional fusion into strain HB8242 which lacks Fur and is Sfp+ (25). This strain constitutively expresses bacillibactin and therefore constitutively activates PfeuA. β-galactosidase activity was assayed in LB medium using a modification of the procedure of Miller (37) as described in Ref. (38).
Purification of RNAP, σA, Btr and BB
RNAP was purified from B. subtilis CU1065 cells by polyethyleneimidine precipitation followed by heparin affinity and size exclusion (Superdex 200 FPLC) column chromatography as previously described (39). The resulting RNAP is a mixture of core enzyme and σA holoenzyme. For reconstitution of σA-saturated holoenzyme, σA was purified after overproduction in E. coli using a DEAE-sepharose column, followed by a monoQ column as described in Ref. (39). His-tagged Btr protein was purified after overproduction in E. coli using Ni-NTA beads followed by size exclusion column chromatography (Superdex 200 FPLC) as described in Ref. (25). Ferric-bacillibactin (here designated as BB) was purified from the supernatant of iron starved cultures of B. subtilis strain (an Sfp+ strain) using a modification of published procedures (40) as described in Ref. (25).
DNA binding (EMSA) and melting (KMnO4) assays
PfeuA containing DNA fragments were amplified from B. subtilis chromosomal DNA by PCR using a [γ-32P]-ATP labeled primer. To monitor Btr binding to PfeuA, EMSA were done using <100 pM of DNA as described previously (25) in a buffer containing: 20 mM Tris pH 8.0, 50 mM NaCl, 50 mM KCl, 5%(v/v) glycerol, 5 µg/ml salmon sperm DNA and 2 mM DTT. For RNAP EMSA, the buffer used was 20 mM Tris–HCl (pH 8.0), 10 mM MgCl2, 2 mM DTT, and 50 mM KCl, 5 µg/ml acetylated BSA, and 5% (v/v) glycerol. A competitor oligonucleotide duplex containing a consensus promoter (Pcon) was formed from oligonucleotide 5′GGCTCTTGACAAAAGTGTTAAATTGTGCTATACTGTATTGGTATGGATGACAGAATTCGG3 ′ and its complement. The two oligonucleotides were mixed together, heated at 95°C for 5 min and annealed at 55°C for 1 min followed by slow cooling to room temperature. DNA was combined with Btr and BB (where indicated) in the reaction buffer and incubated for 5 min at room temperature. RNAP was added and the tubes were incubated at 37°C for 5 min, followed by addition of A, G and U (0.25 mM each) and further incubation for 10 min. Pcon was added to a final concentration of 1.5 µM and the reaction was incubated for 5 min (unless indicated otherwise) and loaded on a 5% polyacrylamide native gel in TBE buffer.
To monitor promoter melting at PfeuA, RNAP and regulatory proteins were added to negatively supercoiled plasmid (pJPM122 carrying PfeuA) and KMnO4 probing and detection of DNA reactivity by primer extension was done as previously described (41). Sites of reactivity were indexed using an A+G chemical sequencing ladder generated from an end-labeled DNA fragment as size markers.
In vitro transcription
For in vitro transcription, σA-saturated holoenzyme was reconstituted by mixing purified RNAP with purified σA (1:5 molar ratio) in transcription buffer (10 mM Tris, 10 mM MgCl2, 0.5 mM EDTA, 1 mM DTT, 10 mM KCl, 50 µg/ml acetylated BSA) and incubating on ice for 15 min. The in vitro transcription reactions contained 100 ng (10 nM) of PfeuA promoter fragment in transcription buffer with Btr or Btr:BB (as indicated) incubated for 15 min at room temperature. For most transcription reactions, a 568-bp PCR product was amplified using primer 1 (5′GCGAAGCTTTGGGGATTTAGGATTCAG3′) and primer 2 (5′GCGGGATCCCGTAATGGCAATTTTGTCT3′) to give a fragment that yields a 224-nt transcript. RNAP was added and the reactions were incubated for 10 min at 37°C. Transcription was initiated by adding 0.25 mM (final concentration) of each nucleotide and 25 µCi of [α-32P]-UTP. After 7-min incubation, the reaction products were ethanol precipitated in the presence of 0.3 M sodium acetate pH 5.2 and 3 µg glycogen. The RNA pellet was washed with 70% cold ethanol, dried and dissolved in formamide containing loading buffer and separated on a 6% denaturing polyacrylamide sequencing gel with a DECADE RNA marker (Ambion). For single round transcription reactions, the same procedure was followed except that CTP was omitted from the NTP mixture. After 10 min of incubation at 37°C, 0.25 mM CTP and 20 µg/ml heparin (final concentrations) were added.
For the abortive transcription assay, a similar protocol was used except that DNA was PCR amplified with primers 1 and 3 (5′-GTAAGAGATATCTTTTTCATCTAT-3′) to give a 417-bp fragment yielding a 55-nt transcript. Reaction products were precipitated in the presence of 0.3 M sodium acetate with glycogen as a carrier and analyzed on a 23% polyacrylamide gel as described in Ref. (42).
RESULTS AND DISCUSSION
Btr binds to a direct repeat in the feuA promoter
We demonstrated previously that Btr protects an extended region in the feuA promoter region (PfeuA) which contains a 9 base direct repeat that overlaps with the upstream portion of the −35 element (25). Btr bound to ferric-bacillibactin (designated Btr:BB) has a high affinity (Kd of ∼16 nM) for PfeuA in vitro and strongly (∼100-fold) activates transcription in vivo. In contrast, in the absence of activating ligand, Btr binds DNA with ∼2-fold reduced affinity in vitro and activates transcription ∼22-fold in vivo (25). Thus, Btr is required for the basal level PfeuA expression (as seen in strains unable to synthesize BB).
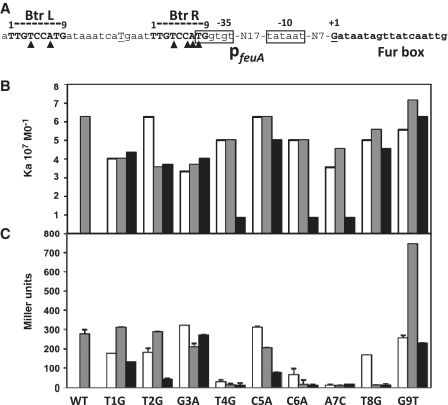
Here, we have sought to define the sequence requirements for Btr binding and activation by introducing a series of mutations into the left and right conserved repeat sequences (Figure 1). Single mutations in any 1 of the 9 bp's of either the left or right arms of the direct repeat led to only modest decreases (<2-fold) in Btr:BB binding affinity as measured by electrophoretic mobility shift assay (EMSA; Figure 1B and Supplementary Figure S1). However, double mutations in both arms at positions 4, 6 or 7 greatly reduced Btr:BB binding affinity with only weak binding detected even at 120 nM (Figure 1B and Supplementary Figure S1). Btr migrates as a dimer during gel exclusion chromatography and, by analogy with well-studied AraC family proteins (43,44), we propose that the two DNA-binding domains within each dimer recognize the two direct repeat sequences. Our results suggest that positions 4, 6 and 7 are critical for Btr:BB binding and that a high affinity DNA–protein complex can form despite mutations in one repeat, but not when both repeats are mutated.
Figure 1.
Characterization of the Btr binding site. (A) Sequence of PfeuA showing the Btr binding site as a 9-bp direct repeat. A capital underlined T between the repeats was changed from G to create a restriction site (for generation of double mutants). The −35 and −10 elements are boxed and are separated by a 17-bp spacer region. The transcription start site is G (marked as +1). Closed triangles show positions critical for Btr:BB-mediated feuA activation. (B) Association constants (Ka) of Btr:BB binding to WT PfeuA (left most gray bar) and PfeuA mutants (single mutation in left repeat shown in white, single mutation in right repeat in gray, and the corresponding double mutation in black) as indicated underneath panel C. In each case, the binding affinity was measured by EMSA in the presence of 0.2 µM BB (see Supplementary Figure S1 for raw data). (C) Expression from PfeuA WT and mutants in vivo was monitored using a PfeuA-lacZ reporter integrated into strain HB8242 which lacks Fur and constitutively expresses BB (25). Mutations are indicated as in panel B (each set of three represents mutations in the left repeat, right repeat and both, respectively).
One caveat with this analysis is that these DNA-binding experiments were conducted in the absence of RNAP. RNAP could increase the affinity of Btr:BB for DNA by favorable protein–protein interactions. Conversely, RNAP could decrease the affinity of Btr:BB for DNA by, for example, competing for a common DNA region such as the right arm of the Btr activator binding site which overlaps with the PfeuA −35 element. Therefore, the requirements for DNA-binding and transcription activation are not necessarily identical.
Btr:BB has stringent sequence requirements for activation of transcription
Next, we monitored the effects of these same activator binding site mutations on the ability of Btr:BB to activate transcription in vivo. As anticipated, those mutations that greatly decreased the affinity of Btr:BB for PfeuA in vitro also prevented activation in vivo as monitored using a PfeuA-lacZ reporter (Figure 1C). Intriguingly, mutations in the right arm at position 4 or 6, or in either arm at position 7, eliminated activation in vivo (Figure 1C), despite the fact that these changes had little effect on Btr:BB affinity for DNA in vitro (Figure 1B).
These findings suggest that (i) Btr binds PfeuA in the context of a functional activation complex in vivo, (ii) this complex has more stringent requirements for formation than detected in an in vitro EMSA analysis (in the absence of RNAP) and (iii) that interaction with the right arm is especially important in the activation mechanism. Note that the right arm of the Btr binding site overlaps the −35 region recognized by σ region 4. This region of PfeuA lacks a strong −35 consensus sequence (TgGtgt has only two matches to the TTGACA consensus). However, support for this assignment is provided by the effects of mutations in this region: mutation of the first (consensus) T to G eliminates activity (T8G in the right repeat; Figure 1C) and mutation of the second G to a consensus base (G9T in the right repeat; Figure 1C) increases activity. This suggests a model in which binding of the downstream protomer of the Btr dimer to the right repeat establishes contacts with RNAP and thereby compensates for a weak −35 element, as previously suggested for AraC (45). To determine which step(s) in transcription initiation are affected by Btr:BB, we next used biochemical analyses diagnostic for promoter binding, RPO formation, transcription initiation and promoter escape.
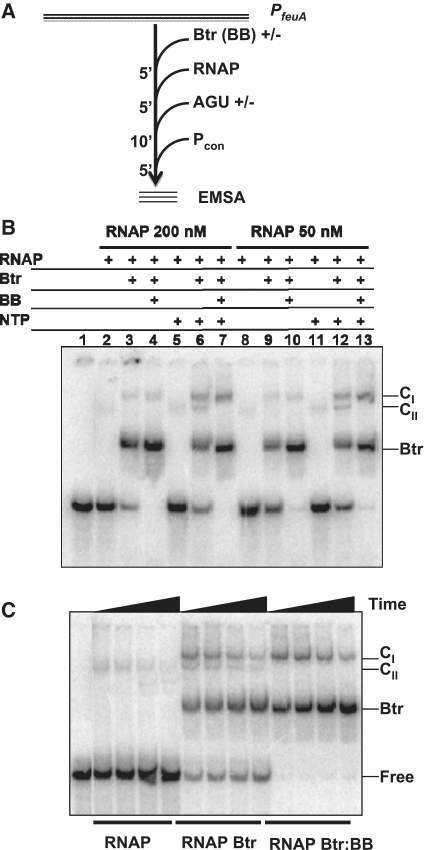
Btr and Btr:BB enhance the formation of competitor resistant RNAP complexes at PfeuA
We first used EMSA to analyze promoter binding. In initial experiments, we noted that RNAP alone forms a high molecular weight complex that was largely retained in the well thereby precluding quantification (data not shown). We therefore conducted subsequent EMSA experiments after addition of a consensus promoter duplex (Pcon) that competes for non-specifically or reversibly bound RNAP (46) as shown in Figure 2A. Under these conditions, only a small amount of complex was detectable after 5 min of Pcon competition (Figure 2B; lanes 2 and 8) with RNAP alone. This is consistent with the fact that most characterized B. subtilis RNAP promoter complexes remain competitor sensitive until after the formation of one or more phosphodiester bonds, presumably because open (RPO) and closed (RPC) complexes are in rapid equilibrium with each other and with free RNAP (47).
Figure 2.
Formation of RNAP complexes at PfeuA. (A). Schematic of the order and timing of addition for EMSA experiments. (B) RNAP complex formation monitored by EMSA. Lane 1. DNA alone. Lanes 2–13. RNAP (100 nM) was incubated with PfeuA in the presence of 50 nM Btr and 0.75 µM BB as indicated for a total of 15 min prior to challenge with 1.5 µM Pcon (competitor duplex DNA containing a consensus promoter). Where indicated (lanes 5–7 and 11–13), 0.25 mM NTPs (ATP, GTP and UTP) were added 10 min prior to Pcon. The mobility of DNA complexes containing only Btr or containing RNAP (±Btr or Btr:BB) are indicated (CI and CII). (C) Stability of CI and CII as a function of time after addition of Pcon. EMSA was done using 100 nM of RNAP in the presence of AGU (as above) and the reactions were analyzed 2.5, 5, 10 or 15 min after addition of Pcon.
Interestingly, if RNAP was incubated with PfeuA in the presence of Btr or Btr:BB, a new complex was detected which migrated with a lower mobility (Figure 2B; lanes 3, 4, 9, 10). We designate this upper complex as CI and suggest that it contains Btr (with or without BB), RNAP and PfeuA DNA. The lower CII complex is similar in mobility to that seen with RNAP alone. Note that the major shifted band detected in these assays is the DNA:Btr complex and this complex increases with time after competitor addition (Figure 2C). Thus, competitor gradually sequesters RNAP from the activator-stabilized RNAP:PfeuA complexes.
We next monitored the effects of adding NTPs on the formation and stability of RNAP:PfeuA complexes. Note that in the absence of Btr, RNAP does not form competitor resistant complexes at PfeuA even in the presence of ATP, GTP and UTP (AGU) which can potentially allow the formation of nascent transcripts up to the 12-mer (Figure 2B; lanes 5 and 11). Indeed, after only 2.5 min of competition nearly all of the complexes are dissociated (free DNA predominates; Figure 2C). In contrast, AGU enhances the formation of the Btr-dependent CI complex (Figure 2B; lanes 6, 7, 12 and 13) and these complexes are relatively stable against Pcon competition with a measured half-life of >8 min (Figure 2C). At both concentrations of RNAP tested, only AGU enhanced the formation of competitor-resistant complexes whereas ATP and GTP, which can allow synthesis only up to the dinucleotide, was insufficient for complex stabilization (data not shown).
We conclude that, even in the presence of Btr:BB and with NTPs allowing synthesis of RNA (up to 12 nt in length), RNAP does not transition to a competitor resistant RPE complex. Instead, we suggest that transiently formed RPITC (containing between 2 and 12 nt RNA transcripts) are in equilibrium with RPO (by release of abortive products), with RPC, and therefore also with free RNAP. This is consistent with prior studies of B. subtilis RNAP in which it has been noted that the early steps in promoter binding and initiation are typically reversible and therefore sensitive to competitors (47–51).
Both Btr and Btr:BB enhance open complex formation
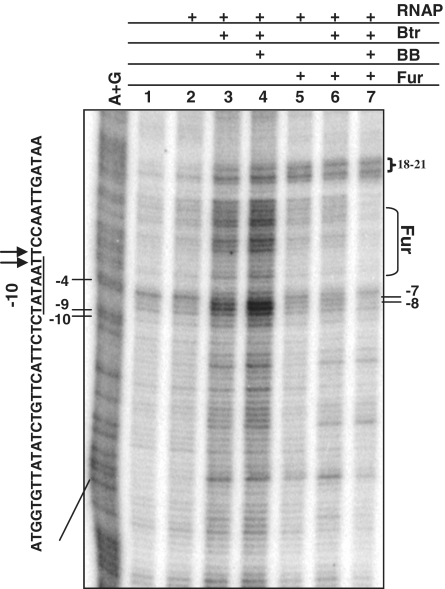
In previous EMSA studies, E. coli RNAP at Plac was observed to form two distinct promoter-bound RPO complexes which, however, differed in their ability to escape from abortive initiation into productive elongation (promoter clearance) (52). We therefore wished to test whether CI and CII, as detected here, are also open complexes as detected using KMnO4 footprinting. In initial experiments, using end-labeled linear DNA fragments, we were unable to detect promoter melting at PfeuA by RNAP under any conditions tested including the presence or absence of Btr, BB and AGU (data not shown). These experiments suggest that even in the presence of Btr:BB and NTPs, RPO and RPITC are transient intermediates in equilibrium with RPC. The lack of KMnO4 reactivity suggests that only low equilibrium levels of RPO and RPITC are present at this promoter.
To increase the ability to detect RPO formation, we repeated these studies using a negatively supercoiled plasmid template. Even with negatively supercoiled DNA, RNAP was unable to form an open complex in the absence of Btr (Figure 3; lane 2). Addition of Btr or Btr:BB only slightly enhanced RPO formation. The level of KMnO4 sensitivity detected at positions −8 to −6 was enhanced only ∼2-fold (after normalization to control bands in the +18 to +21 region) compared to reactions in which Fur was present to prevent RNAP binding (Figure 3; lanes 3 and 4 versus lanes 5–7). Interestingly, both Btr and Btr:BB also led to an increase in KMnO4 reactivity within the initial transcribed region (corresponding to the binding site of Fur). The Fur box sequence has similarity with the −10 consensus for σA (Figure 1A) and this might contribute to this extended pattern of reactivity.
Figure 3.
Effects of Btr and Btr:BB on open complex formation. Open complex (RPO) formation was monitored by KMnO4 footprinting on negatively supercoiled DNA. Lane 1 is an A+G chemical sequencing ladder (for indexing) and lane 2 is a reaction that contains only plasmid DNA. RNAP (100 nM), Btr (50 nM), BB (200 nM) and Fur (50 nM) were added as indicated. Chemical sensitivity within the transcription bubble (e.g. −7,−8) in lanes 3 and 4 was normalized by comparison to the background reactivity in the +18 to +21 region in lanes containing Fur protein (5–7).
We conclude that RNAP fails to efficiently establish a stable RPO complex at PfeuA, and that both Btr or Btr:BB have a modest, but measurable, impact on this step. Since stabilization of RPO appears to be largely independent of BB, we suggest that this may account for the previously reported requirement for Btr for the basal level transcription from PfeuA seen in strains unable to synthesize BB (25). However, full activation of PfeuA requires BB, suggesting that there is a rate-limiting step that is activated in a ligand (BB)-dependent manner in vivo.
BB greatly enhances the ability of Btr to stimulate productive RNA synthesis
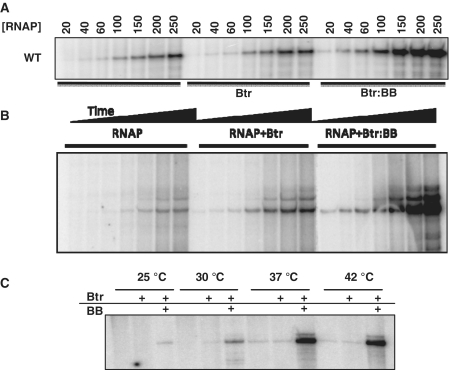
We next used multiple round, in vitro transcription reactions to monitor the effects of Btr and Btr:BB on productive RNA synthesis. In this assay, Btr had a modest effect on RNA yield (<1.5-fold), whereas Btr:BB had a much stronger effect (>5-fold at RNAP concentrations of >50 nM) (Figure 4A). Importantly, this mirrors the BB-dependent transcription activation observed in vivo (25). In a time course experiment (Figure 4B), the rate of RNA accumulation was stimulated 3.4-fold by Btr alone and ∼21-fold by Btr:BB compared to RNAP alone. The magnitude of the activation in this study was somewhat higher than noted in the RNAP titration study (Figure 4A), which may reflect the use of different preparations of both Btr and RNAP.
Figure 4.
Effects of Btr and Btr:BB on in vitro transcription. (A) Multiple round in vitro transcription reactions as a function of RNAP concentration. Btr (50 nM) and BB (200 nM) were added as indicated and the reactions were analyzed using a 6% polyacrylamide denaturing gel. (B) Multiple round in vitro transcription reactions as a function of time. Reactions contained 100 nM RNAP, 50 nM Btr and 200 nM BB. RNAP was allowed bind PfeuA and the reaction was started by addition of NTPs and stopped after 15, 30, 60, 120, 300, 600 and 1200 s. (C) Single round in vitro transcription reaction using 200 nM of RNAP, 50 nM Btr and 200 nM BB. RNAP was incubated with DNA and (as indicated) Btr or Btr:BB, in the presence of ATP, GTP and 32P-UTP for 10 min. CTP was then added together with heparin (final conc. of 20 µg/ml) and the reaction was further incubated for another 10 min.
We reasoned that if Btr:BB function primarily to increase RNAP recruitment, their effects should be most pronounced at low concentrations of RNAP and there should be little if any stimulation at saturating RNAP concentrations. However, in several replicate experiments, the magnitude of stimulation of RNA synthesis by Btr:BB was similar at low and high concentrations of RNAP (Figure 4A and data not shown). This suggests that activation of transcription occurs largely at a concentration-independent step. Since, as noted earlier, RPC and RPO are in rapid equilibrium at many B. subtilis promoters, both promoter melting (RPO formation) and early steps in initiation often partition into the concentration dependent steps in the RNA initiation pathway (47,49,50,53).
The effect of BB on transcription activation by Btr is even more dramatic in single round transcription assays (Figure 4C). In this assay, RNAP was incubated (alone, with Btr or with Btr:BB) in the presence of AGU (which is necessary to stabilize the complexes against heparin) and the resulting complexes were challenged by simultaneous addition of heparin and CTP. Under these conditions, efficient transcription was only observed in the presence of Btr:BB (Figure 4C). Since both Btr and Btr:BB enhance the stable association of RNAP with PfeuA (e.g. the increased yield of CI complexes; Figure 2), and both weakly activate RPO formation (Figure 3), we hypothesized that the nature and equilibrium distribution of the RPITC complexes formed with Btr:BB must differ from that formed with Btr alone. This could, for example, be explained by differences in the abortive initiation properties of the RNAP:Btr and RNAP:Btr:BB complexes.
To monitor abortive initiation products under reaction conditions allowing productive RNA synthesis, we performed transcription using a PfeuA fragment that produces a 55-nt run-off transcript to be able to resolve both full and abortive transcripts on the same gel (Figure 5). Surprisingly, both RNAP alone and in the presence of Btr produced abortive transcripts up to and including the U9 product (the 9-nt product terminating with U). However, in the presence of Btr:BB, RNAP produced longer abortive transcripts (up to 12 nt) and a 3.5- to 4-fold greater amount of the full-length 55-nt transcript, as expected (Figure 5; see inset). Chase experiments using high concentrations of NTPs did not affect the level of abortive transcripts, suggesting that these are not due to RNAP pausing (data not shown). The very similar levels of abortive transcription products (≤9 nt) seen in all three conditions (RNAP, RNAP plus Btr and RNAP plus Btr:BB) supports a model in which Btr:BB acts primarily on a late stage in transcription initiation to enhance the escape from abortive synthesis. In the single round transcription reactions, Btr:BB enables a transition from synthesis of abortive products of 9 nt or less (in the RNAP and RNAP plus Btr lanes) to a longer RPITC complex (up to 12 nt) that can efficiently escape the promoter to yield a full length product (Figure 4C).
Figure 5.

Effects of Btr and Btr:BB on abortive and productive transcription. Abortive and productive transcription products from the same reaction were separated on a 23% polyacrylamide gel. The upper inset is a shorter exposure of the same gel to more clearly show the ∼3.5- to 4-fold increase in the level of productive transcripts with Btr:BB. Lane 1 is the no protein control; lanes 2–4 with 200 nM RNAP, lanes 3 and 4 contain 50 nM Btr and lane 4 with 0.2 µM BB.
Activator binding site mutations affect RNAP complex isomerization and productive transcription
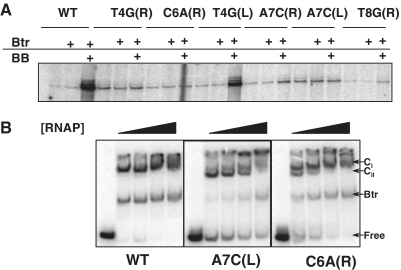
Our mutational studies (Figure 1) suggest that the ability of Btr:BB to activate transcription requires a precise positioning of the activator relative to the RNAP:promoter complex. Next, we used single round transcription reactions to test the effects of these activator binding site mutations on RNA synthesis. Indeed, five of the six tested binding site mutations greatly decreased productive RNA synthesis even in reactions containing Btr:BB (Figure 6A). The one exception was a T4G mutation in the promoter distal (left) arm of the Btr binding site. Thus, there is an excellent correlation between the ability of the mutant activator binding sites to respond to Btr:BB in vivo and their ability to support conversion of RNAP to a heparin-resistant RPITC complex in vitro.
Figure 6.
Effects of PfeuA mutations on RNAP engagement and in vitro transcription. (A) Single round transcription assay of PfeuA WT and various activator binding site mutants. Reactions were done under identical conditions as in Figure 4C. As seen in Figure 4C, high yields of the run-off transcript are only observed in the presence of Btr:BB. The ability of Btr:BB to activate transcription is severely compromised by the T4G(R), C6A(R), A7C(R), A7C(L) and T8G(R) mutations, but only modestly affected by the T4G(L) mutation. For each mutation, the nomenclature indicates the base in the wild-type, the position within the direct repeat, the substituted base and whether the mutation is in the left (L) or right (R) repeat as shown in Figure 1. (B) EMSA analysis of the PfeuA WT and the A7C(L) and C6A(R) activator binding site mutants using different concentrations of RNAP (50, 100, 150 and 200 nM), 50 nM Btr, and 200 nM BB. RNAP complexes CI and CII are indicated. In each set, the first lane represents DNA alone.
One trivial reason why these activator binding site mutations might prevent transcription activation would be an inability to bind Btr:BB. As noted earlier, the affinity for Btr:BB was measured in the absence of RNAP (Figure 1B). To test this idea, we monitored the effects of Btr:BB on RNAP complex formation at PfeuA using EMSA. As above, the binding reactions contained AGU (to allow formation of RPITC) and 50, 100, 150 or 200 nM of RNAP and the complexes were challenged with Pcon (Figure 6B). The results indicate that RNAP can be efficiently engaged at the promoter by Btr:BB despite the activator binding site mutations, but the nature of the resulting complexes differs. While the CI complex forms at high levels with 50 nM RNAP on wild-type PfeuA, DNA containing the A7C mutation in the left arm [designated A7C(L)] formed mainly the CII complex and can only achieve 50% of CI complex even at 150 nM RNAP (Figure 6B). Similarly, the C6A(R) mutant DNA also required higher levels of RNAP to form the CI complex when compared to the wild-type activator binding site (Figure 6B). Collectively, these results suggest that Btr:BB facilitates formation of a complex (CI) that more efficiently escapes from abortive cycling to enable productive RNA synthesis.
A model for Btr:BB-dependent transcription activation
Here, we define the binding site required for activation of transcription by Btr:BB and propose a model for the ligand-stimulated activation of PfeuA transcription. The dimeric Btr:BB binds to a 9 base direct repeat in which the right (promoter proximal) element is juxtaposed to the PfeuA −35 element. In EMSA studies, the simultaneous mutation of both arms of the direct repeat (at positions 4, 6 or 7) was required to substantially impair Btr binding (Figure 1B). In contrast, single point mutations in the right arm at any of these positions was sufficient to prevent Btr:BB activation of feuA expression in vivo (Figure 1C) and in vitro in single round transcription reactions (Figure 6A). We interpret this to suggest that Btr:BB must be precisely positioned relative to RNAP for activation of transcription. This is supportive of a model in which the downstream Btr protomer must make specific contacts with RNAP (perhaps with the σ subunit) that serve to compensate for the weak −35 element.
EMSA analyses reveal that RNAP can form two complexes with either Btr or Btr:BB at PfeuA, CI and CII (Figure 2). Btr:BB enhances the formation of the CI complex (Figures 2B and 6B), productive transcription (Figure 4C), and the formation of longer abortive transcripts (Figure 5). The correlation between the formation of a relatively stable CI complex and productive transcription is supported by the analysis of mutant activator binding sites: mutations that reduced the fraction of CI complexes (Figure 6B) also reduced transcription activation both in vivo (Figure 1C) and in vitro (Figure 6A). Although the precise nature of the CI complex remains to be established, we suggest that Btr:BB specifically alters the nature of the RPO/RPITC complexes to favor promoter escape and productive transcription. Thus, ligand (BB) responsive transcription activation results primarily from enhanced promoter clearance (Figure 7). In contrast, either Btr or Btr:BB can help stabilize RNAP binding to the promoter (Figure 2B and C), and both may weakly stimulate RPO formation (Figure 3). These effects may account for the previously noted requirement for Btr for basal level transcription of PfeuA as seen in strains unable to synthesize BB (25).
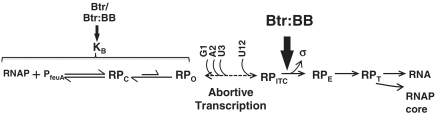
Figure 7.
Model for the ligand-dependent stimulation of the PfeuA promoter clearance by Btr:BB. RNAP binds reversibly to PfeuA to generate an initial closed complex (RPC) which isomerizes (perhaps through undetected intermediates) to an open complex (RPO). Permanganate footprinting studies (Figure 3) suggest that RPO forms inefficiently at PfeuA, even in the presence of Btr or Btr:BB, as indicated by the relative arrow sizes. Btr and Btr:BB have a modest stimulatory effect on RNAP:PfeuA complex formation (as detected in EMSA assays; Figure 2) and Btr alone weakly stimulates transcription in multiple round reactions (Figure 4B). We suggest that this is due to an increase in KB (perhaps due to a decrease in the rate of RNAP dissociation due to stabilizing Btr:RNAP contacts). The major effect of Btr:BB on transcription activation is assigned to the promoter clearance step. Specifically, Btr:BB increases full-length RNA production while having no detectable affect on the yield of short (<10 nt) abortive products (Figure 5).
Comparison with other AraC activators
Members of the AraC family typically contain two domains: an ∼100 amino acid C-terminal DNA binding domain (54) and an additional N-terminal domain that mediates dimerization and substrate binding (55). These proteins regulate diverse functions including sugar catabolism, responses to stress and virulence (55). Btr, in contrast, has an AraC-like domain (comparable in size to most full length AraC proteins) which is joined to a C-terminal BB binding domain. The BB binding domain is homologous to the substrate-binding protein (FeuA) involved in ferric-BB uptake and confers ligand responsiveness (25).
Mechanisms of activation by AraC family members include RNAP recruitment and open complex stimulation as shown in E. coli for the regulation of araBAD by AraC (44,56). AraC family proteins often bind adjacent to or overlapping the −35 region and interact with RNAP. Indeed, E. coli AraC and σ recognize overlapping nucleotides (45). Escherichia coli RhaR and RhaS directly contact domain 4 of σ70 (57–59), whereas activation of Vibrio cholerae tcpA by ToxT requires the alpha C-terminal domain of RNAP (60). At least partly because of the difficulty in purification of AraC family proteins, detailed biochemical insights into the mechanisms of activation have been slow to emerge.
Transcription activation by Btr also appears to involve a precise positioning of the activator protein immediately upstream of bound RNAP. While we do not yet know the molecular details of the interactions between Btr and RNAP, we have here defined the functional consequences of this interaction. Our results suggest a primary, ligand-dependent mechanism that involves an increase in RNAP escape from an abortively transcribing RPITC to a productive elongation complex. While it is well documented that many activators stimulate transcription by RNAP recruitment (61) or by enhancing open complex formation (62,63), activation of promoter clearance has rarely been documented. One recent example is the phage Mu transcription activator C which contacts the ß’ subunit of RNAP to reduce abortive transcription and thereby facilitate promoter clearance at the Mu mom promoter (64). Given the prevalence of AraC-like regulators it will be interesting to determine if activation of promoter clearance is a common activation mechanism within this family of proteins.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Figure 1.
FUNDING
National Institutes of Health (grant GM-059323 to J.D.H.). Funding for open access charge: National Institutes of Health (grant GM-059323 to J.D.H.).
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Lilian Hsu and Irina Artsimovitch for helpful comments on this work.
REFERENCES
- 1.Saecker RM, Record MT, Jr, Dehaseth PL. Mechanism of bacterial transcription initiation: RNA polymerase-promoter binding, isomerization to initiation-competent open complexes, and initiation of RNA synthesis. J. Mol. Biol. 2011;412:754–771. doi: 10.1016/j.jmb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu LM. Promoter clearance and escape in prokaryotes. Biochim. Biophys. Acta. 2002;1577:191–207. doi: 10.1016/s0167-4781(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 3.Hsu LM. Monitoring abortive initiation. Methods. 2009;47:25–36. doi: 10.1016/j.ymeth.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borukhov S, Nudler E. RNA polymerase: the vehicle of transcription. Trends Microbiol. 2008;16:126–134. doi: 10.1016/j.tim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Paget MS, Helmann JD. The sigma70 family of sigma factors. Genome Biol. 2003;4:203. doi: 10.1186/gb-2003-4-1-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruber TM, Gross CA. Assay of Escherichia coli RNA polymerase: sigma-core interactions. Methods Enzymol. 2003;370:206–212. doi: 10.1016/S0076-6879(03)70018-4. [DOI] [PubMed] [Google Scholar]
- 7.Rojo F. Repression of transcription initiation in bacteria. J. Bacteriol. 1999;181:2987–2991. doi: 10.1128/jb.181.10.2987-2991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minchin SD, Busby SJ. Analysis of mechanisms of activation and repression at bacterial promoters. Methods. 2009;47:6–12. doi: 10.1016/j.ymeth.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Ishihama A. Role of the RNA polymerase alpha subunit in transcription activation. Mol. Microbiol. 1992;6:3283–3288. doi: 10.1111/j.1365-2958.1992.tb02196.x. [DOI] [PubMed] [Google Scholar]
- 10.Dove SL. Studying protein-protein interactions using a bacterial two-hybrid system. Methods Mol. Biol. 2003;205:251–265. doi: 10.1385/1-59259-301-1:251. [DOI] [PubMed] [Google Scholar]
- 11.Ross W, Gourse RL. Analysis of RNA polymerase-promoter complex formation. Methods. 2009;47:13–24. doi: 10.1016/j.ymeth.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasse-Dwight S, Gralla JD. KMnO4 as a probe for lac promoter DNA melting and mechanism in vivo. J. Biol. Chem. 1989;264:8074–8081. [PubMed] [Google Scholar]
- 13.Roberts JW, Shankar S, Filter JJ. RNA polymerase elongation factors. Annu. Rev. Microbiol. 2008;62:211–233. doi: 10.1146/annurev.micro.61.080706.093422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menendez M, Kolb A, Buc H. A new target for CRP action at the malT promoter. EMBO J. 1987;6:4227–4234. doi: 10.1002/j.1460-2075.1987.tb02771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tagami H, Aiba H. Role of CRP in transcription activation at Escherichia coli lac promoter: CRP is dispensable after the formation of open complex. Nucleic Acids Res. 1995;23:599–605. doi: 10.1093/nar/23.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichenberger P, Dethiollaz S, Buc H, Geiselmann J. Structural kinetics of transcription activation at the malT promoter of Escherichia coli by UV laser footprinting. Proc. Natl Acad. Sci. USA. 1997;94:9022–9027. doi: 10.1073/pnas.94.17.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts JW, Yarnell W, Bartlett E, Guo J, Marr M, Ko DC, Sun H, Roberts CW. Antitermination by bacteriophage lambda Q protein. Cold Spring Harb. Symp. Quant. Biol. 1998;63:319–325. doi: 10.1101/sqb.1998.63.319. [DOI] [PubMed] [Google Scholar]
- 18.Perdue SA, Roberts JW. sigma(70)-dependent transcription pausing in Escherichia coli. J. Mol. Biol. 2011;412:782–792. doi: 10.1016/j.jmb.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Bar-Nahum G, Nudler E. Isolation and characterization of sigma(70)-retaining transcription elongation complexes from Escherichia coli. Cell. 2001;106:443–451. doi: 10.1016/s0092-8674(01)00461-5. [DOI] [PubMed] [Google Scholar]
- 20.Deighan P, Pukhrambam C, Nickels BE, Hochschild A. Initial transcribed region sequences influence the composition and functional properties of the bacterial elongation complex. Genes Dev. 2011;25:77–88. doi: 10.1101/gad.1991811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artsimovitch I. Post-initiation control by the initiation factor sigma. Mol. Microbiol. 2008;68:1–3. doi: 10.1111/j.1365-2958.2008.06136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusuya Y, Kurokawa K, Ishikawa S, Ogasawara N, Oshima T. Transcription factor GreA contributes to resolving promoter-proximal pausing of RNA polymerase in Bacillus subtilis cells. J. Bacteriol. 2011;193:3090–3099. doi: 10.1128/JB.00086-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Gilmour DS. Promoter proximal pausing and the control of gene expression. Curr. Opin. Genet. Dev. 2011;21:231–235. doi: 10.1016/j.gde.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nechaev S, Adelman K. Pol II waiting in the starting gates: regulating the transition from transcription initiation into productive elongation. Biochim. Biophys. Acta. 2011;1809:34–45. doi: 10.1016/j.bbagrm.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaballa A, Helmann JD. Substrate induction of siderophore transport in Bacillus subtilis mediated by a novel one-component regulator. Mol. Microbiol. 2007;66:164–173. doi: 10.1111/j.1365-2958.2007.05905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egan SM. Growing repertoire of AraC/XylS activators. J. Bacteriol. 2002;184:5529–5532. doi: 10.1128/JB.184.20.5529-5532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schleif R. AraC protein: a love-hate relationship. Bioessays. 2003;25:274–282. doi: 10.1002/bies.10237. [DOI] [PubMed] [Google Scholar]
- 28.Schleif R. AraC protein, regulation of the l-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action. FEMS Microbiol. Rev. 2010;34:779–796. doi: 10.1111/j.1574-6976.2010.00226.x. [DOI] [PubMed] [Google Scholar]
- 29.Griffith KL, Wolf RE., Jr Systematic mutagenesis of the DNA binding sites for SoxS in the Escherichia coli zwf and fpr promoters: identifying nucleotides required for DNA binding and transcription activation. Mol. Microbiol. 2001;40:1141–1154. doi: 10.1046/j.1365-2958.2001.02456.x. [DOI] [PubMed] [Google Scholar]
- 30.Griffith KL, Wolf RE., Jr A comprehensive alanine scanning mutagenesis of the Escherichia coli transcriptional activator SoxS: identifying amino acids important for DNA binding and transcription activation. J. Mol. Biol. 2002;322:237–257. doi: 10.1016/s0022-2836(02)00782-9. [DOI] [PubMed] [Google Scholar]
- 31.Michel L, Gonzalez N, Jagdeep S, Nguyen-Ngoc T, Reimmann C. PchR-box recognition by the AraC-type regulator PchR of Pseudomonas aeruginosa requires the siderophore pyochelin as an effector. Mol. Microbiol. 2005;58:495–509. doi: 10.1111/j.1365-2958.2005.04837.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, James LP, Helmann JD. Metalloregulation in Bacillus subtilis: isolation and characterization of two genes differentially repressed by metal ions. J. Bacteriol. 1993;175:5428–5437. doi: 10.1128/jb.175.17.5428-5437.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Russel DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- 34.Cutting SM, VanderHorn PB. Molecular Biological Methods for Bacillus. Chichester: John Wiley and Sons, Ltd; 1990. Genetic Analysis. [Google Scholar]
- 35.Slack FJ, Mueller JP, Sonenshein AL. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J. Bacteriol. 1993;175:4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuber P, Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 38.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann JD. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 1998;29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 39.Helmann JD. Purification of Bacillus subtilis RNA polymerase and associated factors. Methods Enzymol. 2003;370:10–24. doi: 10.1016/S0076-6879(03)70002-0. [DOI] [PubMed] [Google Scholar]
- 40.Dertz EA, Stintzi A, Raymond KN. Siderophore-mediated iron transport in Bacillus subtilis and Corynebacterium glutamicum. J. Biol. Inorg. Chem. 2006;11:1087–1097. doi: 10.1007/s00775-006-0151-4. [DOI] [PubMed] [Google Scholar]
- 41.Maclellan SR, Wecke T, Helmann JD. A previously unidentified sigma factor and two accessory proteins regulate oxalate decarboxylase expression in Bacillus subtilis. Mol. Microbiol. 2008;69:954–967. doi: 10.1111/j.1365-2958.2008.06331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsu LM. Quantitative parameters for promoter clearance. Methods Enzymol. 1996;273:59–71. doi: 10.1016/s0076-6879(96)73006-9. [DOI] [PubMed] [Google Scholar]
- 43.Grainger DC, Belyaeva TA, Lee DJ, Hyde EI, Busby SJ. Binding of the Escherichia coli MelR protein to the melAB promoter: orientation of MelR subunits and investigation of MelR-DNA contacts. Mol. Microbiol. 2003;48:335–348. doi: 10.1046/j.1365-2958.2003.t01-1-03434.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Reeder T, Schleif R. Transcription activation parameters at ara pBAD. J. Mol. Biol. 1996;258: 14–24. doi: 10.1006/jmbi.1996.0230. [DOI] [PubMed] [Google Scholar]
- 45.Dhiman A, Schleif R. Recognition of overlapping nucleotides by AraC and the sigma subunit of RNA polymerase. J. Bacteriol. 2000;182:5076–5081. doi: 10.1128/jb.182.18.5076-5081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaal T, Ross W, Estrem ST, Nguyen LH, Burgess RR, Gourse RL. Promoter recognition and discrimination by Eσs RNA polymerase. Mol. Microbiol. 2001;42:939–954. doi: 10.1046/j.1365-2958.2001.02703.x. [DOI] [PubMed] [Google Scholar]
- 47.Whipple FW, Sonenshein AL. Mechanism of initiation of transcription by Bacillus subtilis RNA polymerase at several promoters. J. Mol. Biol. 1992;223:399–414. doi: 10.1016/0022-2836(92)90660-c. [DOI] [PubMed] [Google Scholar]
- 48.Seredick SD, Spiegelman GB. The Bacillus subtilis response regulator Spo0A stimulates sigmaA-dependent transcription prior to the major energetic barrier. J. Biol. Chem. 2004;279:17397–17403. doi: 10.1074/jbc.M311190200. [DOI] [PubMed] [Google Scholar]
- 49.Juang YL, Helmann JD. Pathway of promoter melting by Bacillus subtilis RNA polymerase at a stable RNA promoter: effects of temperature, delta protein, and sigma factor mutations. Biochemistry. 1995;34:8465–8473. doi: 10.1021/bi00026a030. [DOI] [PubMed] [Google Scholar]
- 50.Rojo F, Nuez B, Mencia M, Salas M. The main early and late promoters of Bacillus subtilis phage phi 29 form unstable open complexes with sigma A-RNA polymerase that are stabilized by DNA supercoiling. Nucleic Acids Res. 1993;21:935–940. doi: 10.1093/nar/21.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang BY, Shyu YT, Doi RH. The interaction between Bacillus subtilis sigma-A (sigma A) factor and RNA polymerase with promoters. Biochimie. 1992;74:601–612. doi: 10.1016/0300-9084(92)90131-w. [DOI] [PubMed] [Google Scholar]
- 52.Straney DC, Crothers DM. Intermediates in transcription initiation from the E. coli lac UV5 promoter. Cell. 1985;43:449–459. doi: 10.1016/0092-8674(85)90175-8. [DOI] [PubMed] [Google Scholar]
- 53.Dobinson KF, Spiegelman GB. Effect of the delta subunit of Bacillus subtilis RNA polymerase on initiation of RNA synthesis at two bacteriophage phi 29 promoters. Biochemistry. 1987;26:8206–8213. doi: 10.1021/bi00399a028. [DOI] [PubMed] [Google Scholar]
- 54.Martin RG, Rosner JL. The AraC transcriptional activators. Curr. Opin. Microbiol. 2001;4:132–137. doi: 10.1016/s1369-5274(00)00178-8. [DOI] [PubMed] [Google Scholar]
- 55.Gallegos MT, Schleif R, Bairoch A, Hofmann K, Ramos JL. Arac/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 1997;61:393–410. doi: 10.1128/mmbr.61.4.393-410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schleif R. DNA looping. Science. 1988;240:127–128. doi: 10.1126/science.3353710. [DOI] [PubMed] [Google Scholar]
- 57.Wickstrum JR, Egan SM. Amino acid contacts between sigma 70 domain 4 and the transcription activators RhaS and RhaR. J. Bacteriol. 2004;186:6277–6285. doi: 10.1128/JB.186.18.6277-6285.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhende PM, Egan SM. Genetic evidence that transcription activation by RhaS involves specific amino acid contacts with sigma 70. J. Bacteriol. 2000;182:4959–4969. doi: 10.1128/jb.182.17.4959-4969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Egan SM, Pease AJ, Lang J, Li X, Rao V, Gillette WK, Ruiz R, Ramos JL, Wolf RE., Jr Transcription activation by a variety of AraC/XylS family activators does not depend on the class II-specific activation determinant in the N-terminal domain of the RNA polymerase alpha subunit. J. Bacteriol. 2000;182:7075–7077. doi: 10.1128/jb.182.24.7075-7077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hulbert RR, Taylor RK. Mechanism of ToxT-dependent transcriptional activation at the Vibrio cholerae tcpA promoter. J. Bacteriol. 2002;184:5533–5544. doi: 10.1128/JB.184.20.5533-5544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ptashne M, Gann A. Transcriptional activation by recruitment. Nature. 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 62.Lawson CL, Swigon D, Murakami KS, Darst SA, Berman HM, Ebright RH. Catabolite activator protein: DNA binding and transcription activation. Curr. Opin. Struct. Biol. 2004;14:10–20. doi: 10.1016/j.sbi.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schumacher J, Joly N, Rappas M, Zhang X, Buck M. Structures and organisation of AAA+ enhancer binding proteins in transcriptional activation. J. Struct. Biol. 2006;156:190–199. doi: 10.1016/j.jsb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Swapna G, Chakraborty A, Kumari V, Sen R, Nagaraja V. Mutations in beta’ subunit of Escherichia coli RNA polymerase perturb the activator polymerase functional interaction required for promoter clearance. Mol. Microbiol. 2011;80:1169–1185. doi: 10.1111/j.1365-2958.2011.07636.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.