Abstract
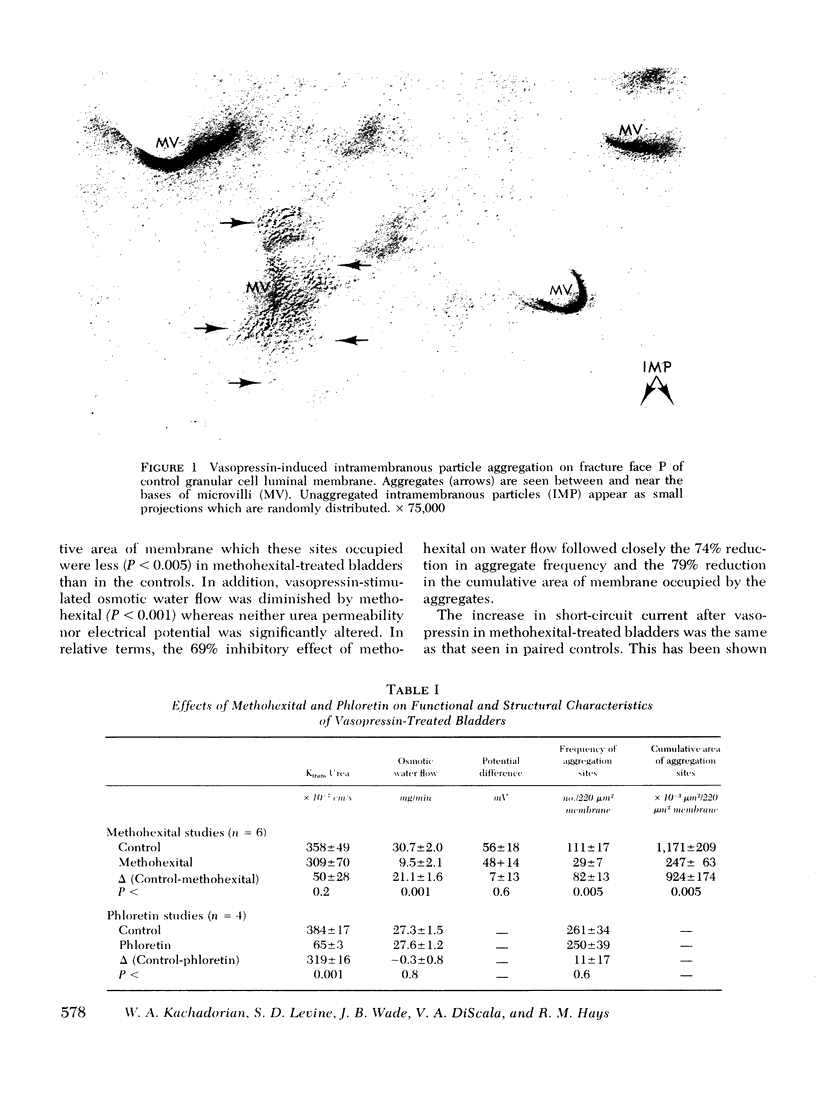
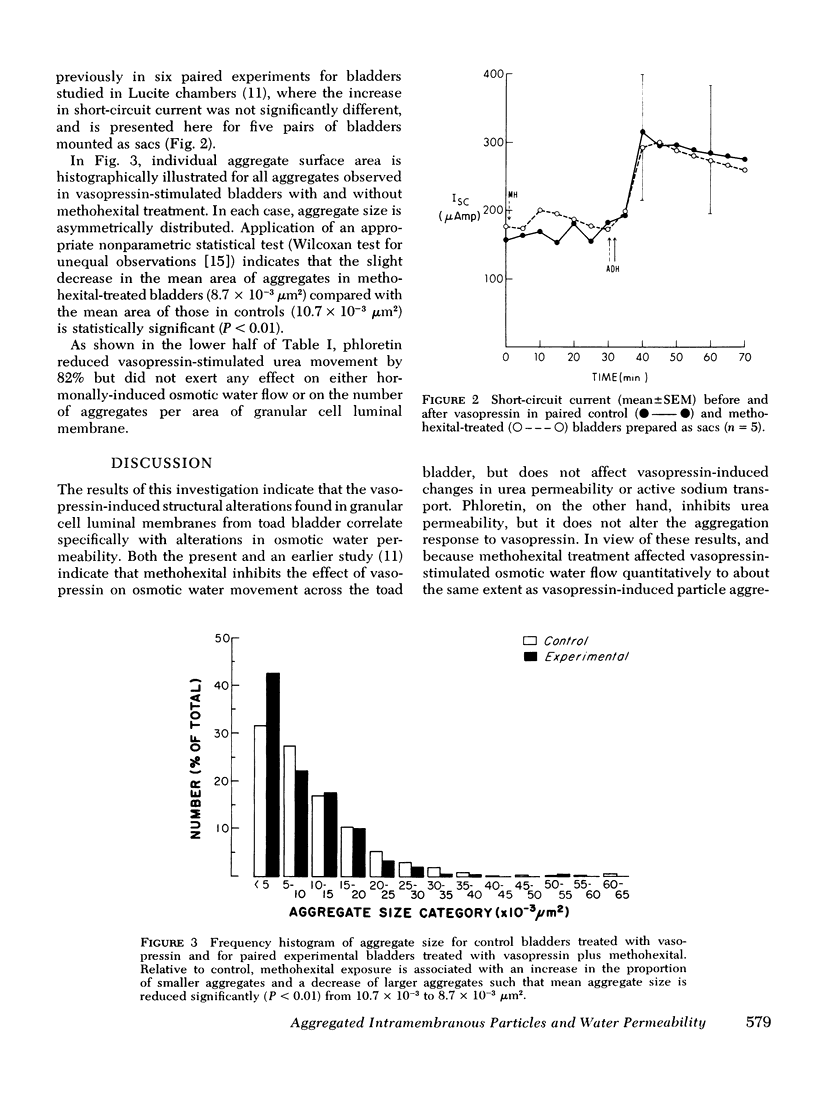
It has been previously demonstrated with freeze-fracture electron microscopy that vasopressin induces specific structural alterations of the luminal membrane of granular cells from toad urinary bladder in a dose-dependent fashion. These alterations consist of aggregated intramembranous particles and are observed both in the presence and absence of an osmotic gradient. We examined the effect of methohexital, a selective inhibitor of vasopressin-stimulated water flow, and the effect of phloretin, a selective inhibitor of urea permeability, on the structure of the granular cell luminal membrane. Methohexital treatment of the vasopressin-stimulated toad bladder reduced both the osmotic water flow and vasopressin-induced alterations of membrane structure to the same extent. Phloretin reduced urea permeability but not water flow or particle aggregation. Since neither agent affects vasopressin-stimulated sodium movement, these findings indicate that the phenomenon of particle aggregation is specifically related to vasopressin-induced water permeability and not to changes in urea or sodium permeability.
Full text
PDF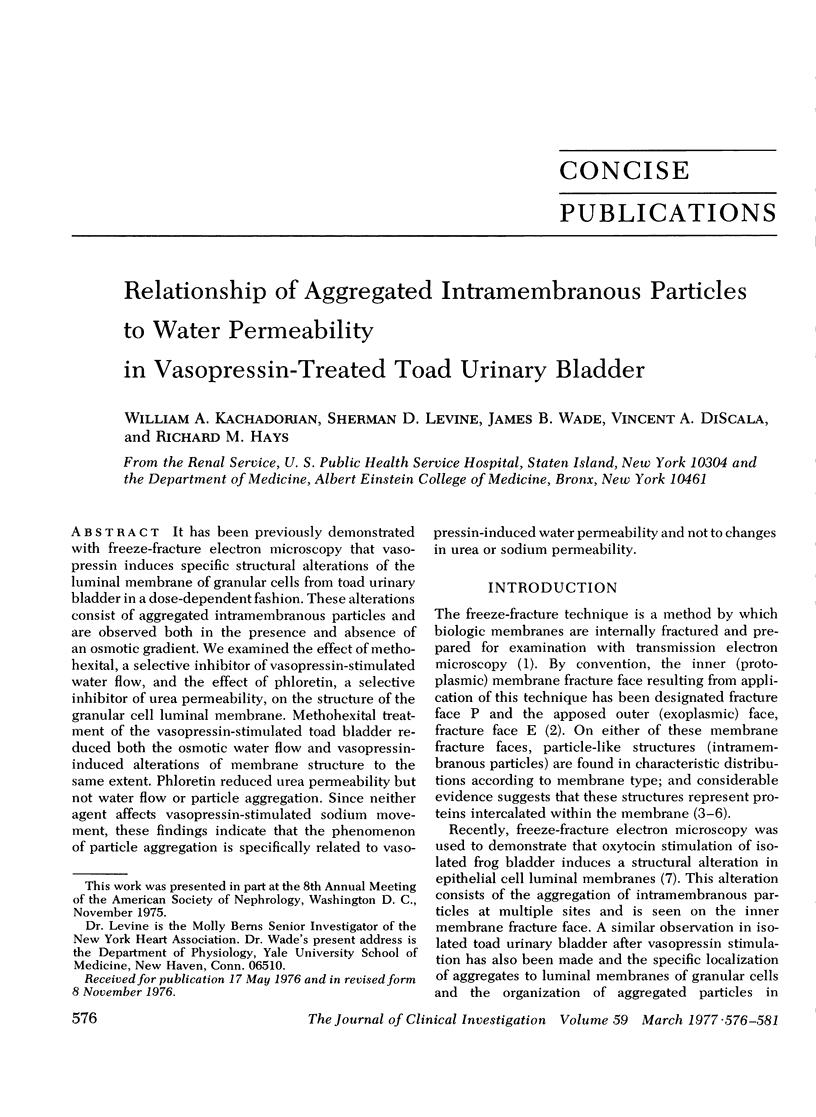



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENTLEY P. J. The effects of neurohypophysial extracts on the water transfer across the wall of the isolated urinary bladder of the toad Bufo marinus. J Endocrinol. 1958 Sep;17(3):201–209. doi: 10.1677/joe.0.0170201. [DOI] [PubMed] [Google Scholar]
- Bourguet J., Chevalier J., Hugon J. S. Alterations in membrane-associated particle distribution during antidiuretic challenge in frog urinary bladder epithelium. Biophys J. 1976 Jun;16(6):627–639. doi: 10.1016/S0006-3495(76)85717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Branton D. Fracture faces of frozen membranes. Proc Natl Acad Sci U S A. 1966 May;55(5):1048–1056. doi: 10.1073/pnas.55.5.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier J., Bourguet J., Hugon J. S. Membrane associated particles: distribution in frog urinary bladder epithelium at rest and after oxytocin treatment. Cell Tissue Res. 1974;152(2):129–140. doi: 10.1007/BF00224690. [DOI] [PubMed] [Google Scholar]
- Civan M. M., DiBona D. Pathways for movement of ions and water across toad urinary bladder. II. Site and mode of action of vasopressin. J Membr Biol. 1974;19(3):195–220. doi: 10.1007/BF01869978. [DOI] [PubMed] [Google Scholar]
- Handler J. S., Butcher R. W., Sutherland E. W., Orloff J. The effect of vasopressin and of theophylline on the concentration of adenosine 3',5'-phosphate in the urinary bladder of the toad. J Biol Chem. 1965 Nov;240(11):4524–4526. [PubMed] [Google Scholar]
- Kachadorian W. A., Wade J. B., DiScala V. A. Vasopressin: induced structural change in toad bladder luminal membrane. Science. 1975 Oct 3;190(4209):67–69. doi: 10.1126/science.809840. [DOI] [PubMed] [Google Scholar]
- Kachadorian W. A., Wade J. B., Uiterwyk C. C., DiScala V. A. Membrane structural and functional responses to vasopressin in toad bladder. J Membr Biol. 1977 Jan 28;30(4):381–401. doi: 10.1007/BF01869678. [DOI] [PubMed] [Google Scholar]
- Levine S. D., Levine R. D., Worthington R. E., Hays R. M. Selective inhibition of osmotic water flow by general anesthetics to toad urinary bladder. J Clin Invest. 1976 Oct;58(4):980–988. doi: 10.1172/JCI108552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Franki N., Hays R. M. Effect of phloretin on water and solute movement in the toad bladder. J Clin Invest. 1973 Jun;52(6):1435–1442. doi: 10.1172/JCI107317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D. H., Seeman P., Iles G. H., Yip C. C. Membrane formation by the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1971 Apr 25;246(8):2702–2710. [PubMed] [Google Scholar]
- ORLOFF J., HANDLER J. S. The similarity of effects of vasopressin, adenosine-3',5'-phosphate (cyclic AMP) and theophylline on the toad bladder. J Clin Invest. 1962 Apr;41:702–709. doi: 10.1172/JCI104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Douglas S. D., Branton D. Localization of A antigen sites on human erythrocyte ghosts. Nature. 1971 Jul 16;232(5307):194–196. doi: 10.1038/232194a0. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P. Membrane intercalated particles in human erythrocyte ghosts: sites of preferred passage of water molecules at low temperature. Proc Natl Acad Sci U S A. 1973 May;70(5):1339–1343. doi: 10.1073/pnas.70.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A., Maffly R., Wilson L., Reaven E. Evidence for involvement of microtubules in the action of vasopressin. Ann N Y Acad Sci. 1975 Jun 30;253:723–737. doi: 10.1111/j.1749-6632.1975.tb19241.x. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourtellotte M. E., Zupnik J. S. Freeze-fractured Acholeplasma laidlawii membranes: nature of particles observed. Science. 1973 Jan 5;179(4068):84–86. doi: 10.1126/science.179.4068.84. [DOI] [PubMed] [Google Scholar]
- Walser M., Butler S. E., Hammond V. Reversible stimulation of sodium transport in the toad bladder by stretch. J Clin Invest. 1969 Sep;48(9):1714–1723. doi: 10.1172/JCI106137. [DOI] [PMC free article] [PubMed] [Google Scholar]




