Alzheimer's disease (AD) has long been in the public eye because of its prevalence in the geriatric population, and the fear that the cognitive haze of dementia will strike us. The pathophysiology of AD is thought to derive from a small peptide, termed Aβ, which accumulates in the brain causing neurotoxicity and neurodegeneration. There is accumulating evidence pointing toward a potentially important link between cholesterol, Aβ, and AD. Recent epidemiological studies indicate that the prevalence of AD is reduced among people taking a class of cholesterol lowering medicines, termed HMG-CoA reductase inhibitors (also known as statins), such as simvastatin and lovastatin (1, 2). This work is supported by studies in transgenic mice overexpressing amyloid precursor protein (APP), which is the precursor to Aβ (Fig. 1A). These studies show that cholesterol levels inversely regulate Aβ production and Alzheimer pathology (3). Transgenic APP mice fed high cholesterol diets have more neuritic plaques and higher levels of insoluble Aβ, which is the main component of neuritic plaques. Now, two articles in the current issue of PNAS (4, 5) provide data suggesting how cholesterol might modulate Alzheimer pathology. Both papers study the affects of cholesterol reduction on APP processing and Aβ production. Fassbender et al. (5) use both cell culture and in vivo studies to show that inhibiting cholesterol production reduces Aβ production, and Kojro et al. (4) provide corroborative evidence by showing that inhibiting cholesterol production increases trafficking of APP through the non-amyloidogenic APPsα pathway. Together these papers suggest that inhibiting cholesterol production in the brain might inhibit Aβ production, and reduce the accumulation of Aβ that causes AD.
Figure 1.
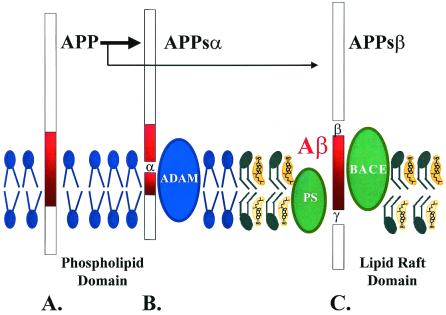
A putative model of the processing of APP in relation to the lipid composition of membranes. (A) APP is a transmembrane protein. (B) Cleavage of APP by α secretases, such as ADAM10, produces APPsα, which requires a membrane domain that is cholesterol poor, such as phospholipid domains. (C) Cleavage of APP by the β and γ secretases, BACE, and Presenilin (PS) produces Aβ and APPsβ. This step requires a membrane domain that is cholesterol rich, such as a lipid raft. APP processing can be directed toward α-secretase or β/γ-secretase pathways by modulating the cholesterol content of the membranes. Lipid identities: blue, phospholipid; green, sphingolipids; yellow, cholesterol.
To understand how and why cholesterol might impact Aβ production, we need to take a step back and understand the mechanisms of Aβ production. Aβ is made in the endoplasmic reticulum as a result cleavage of APP by particular proteases, termed secretases. Cleavage of APP occurs via two paths (Fig. 1). Most APP is cut at the α-secretase site to produce two products, APPsα and a C-terminal fragment (Fig. 1B). The APPsα protein is a neurotrophic protein that is secreted, whereas the C-terminal fragment is internalized and degraded. Cleavage of APP by α-secretase cuts Aβ in half and precludes Aβ production. A small percentage of APP is cleaved by two enzymes, termed β and γ secretases, that lead to production of Aβ (Fig. 1C). Although Aβ is processed from only a small percentage of APP, the pathway producing Aβ is very important because it is responsible for the pathophysiology of AD.
The identity of the enzymes that cleave APP has been the subject of an intense research effort because determining the identity and regulation of these proteins is key to controlling Aβ production. There appears to be one enzyme that predominates at each of the β and γ cleavage sites (Fig. 1C). The predominant enzyme that cleaves APP at the β-secretase site is BACE-1, whereas the predominant enzyme that cleaves the γ-secretase site is presenilin-1 (6, 7). In both cases, there are secondary enzymes, termed BACE-2 and presenilin-2, which can also cleave at this site but are less important in normal processing of APP in neurons. The identity of α-secretase is somewhat more complicated because several different enzymes can cleave APP at this site. The several enzymes that cleave APP at the α-secretase site are ADAM9, ADAM10, and ADAM17 (8–10). The protease ADAM10, which is the subject of the paper by Kojro et al., appears to colocalize best with BACE, suggesting that it is an important α-secretase (11).
The γ-secretase site is particularly unusual because it is an intramembranous cleavage. Intramembranous cleavage appears to be difficult, and perhaps because of this difficulty, the γ-secretase site of APP is ragged. Most Aβ generated by this cleavage is 40 aa in length, but some (about 5%) is 42 aa long. The Aβ42 aggregates very readily, and is thought to form the nidus of plaques in brains of patients with AD (12). Only a few other mammalian proteins cleave at intramembranous sites. The two known examples of proteins cleaved at intramembranous sites are the steroid regulatory element binding protein, which controls cholesterol production, and Notch protein, which controls development (13, 14). The similarity between processing of APP and processing of the steroid regulatory binding protein was one of the first hints that cholesterol might impact on APP processing, although it turns out that the linkage between Notch and APP is more direct because both proteins are cleaved by presenilins (15).
The linkage between cholesterol and APP, although possibly indirect, remains cogent because cholesterol is known to be an important lipid that controls membrane fluidity. Following up on this putative connection, several studies have already shown that reducing cholesterol in neurons reduces Aβ production, but the subject has yet to be investigated in detail (16–18). The papers by Fassbender et al. and Kojro et al. both confirm these prior observations showing that reducing cholesterol reduces Aβ production. Each paper, though, adds significant new insights to the picture. Fassbender et al. take a detailed look at APP processing in primary hippocampal and cortical neurons, and examine each of the species produced during production of Aβ. They show that reducing cholesterol content strongly reduces both Aβ40 and Aβ42. They also show that cholesterol depletion reduces the amount of C-terminal fragment produced by the β-secretase cleavage, which suggests that cholesterol depletion inhibits BACE activity. Kojro et al. examine production of APPsα in detail, focusing on ADAM10, which is one of the major α-secretases. To examine ADAM10 α-secretase activity, they use human embryonic kidney cell lines overexpressing ADAM10, and show that reducing cholesterol increases ADAM10 α-secretase activity. Together, these papers suggest that reducing cholesterol shifts APP processing toward the APPsα pathway and away from production of Aβ by acting coordinately on the multiple enzymes regulating APP processing. This coordination could be serendipitous, or might reflect biochemical integration of the enzymes in the APP processing pathway. The extent of colocalization of the secretases remains unclear because Aβ generation occurs largely in the endoplasmic reticulum, whereas APPsα production occurs both in the endoplasmic reticulum and at the plasma membrane. Processing of APP in the endoplasmic reticulum might occur if the multiple enzymes controlling APP processing work together as part of one large processing unit, such as the large 1 × 106 kDa complex that copurifies with presenilin-1 (6).
It might be possible to develop medicines that target the brain lipids or lipid compartments that specifically regulate Aβ production. This opens up new therapeutic approaches to Alzheimer's disease.
The papers by Fassbender et al. (5) and Kojro et al. (4) also both shed light on the quantitative relationship between cholesterol reduction and inhibition of Aβ production. The prior studies have all used harsh conditions to achieve large reductions in cholesterol, but both of these papers examine the relationship between cholesterol and Aβ under a variety of conditions. Fassbender et al. show that treating neurons with lovastatin or simvastatin alone strongly reduces Aβ production. The reduction is also strong in vivo. They show that guinea pigs treated with simvastatin show up to a 50% decrease in Aβ production in 3 weeks. A note of caution is worthwhile before overinterpreting this result, because the dose of simvastatin used for their study is 200–400 times greater than that used in humans. Although not ideal, high doses of drugs are often required to see effects over the short time frames used in many experiments, and 3 weeks is a short time span relative to the 6-month half-life of cholesterol in the brain (19). The observation that simvastatin reduces Aβ in the brain in vivo is important because it supports the retrospective clinical evidence suggesting that patients taking lovastatin or simvastatin have a reduced risk of AD (1, 2). The mechanism of risk reduction is unknown, but Fassbender's study directs attention toward Aβ and suggests that the mechanism by which statins reduce the risk of AD could derive from reduced production of Aβ in the brain.
These studies also shed light on the complexity of cholesterol biochemistry, and raise important questions about which lipid changes are most critical for reducing Aβ production. Cholesterol turnover in the brain is much slower than in the rest of the body. Studies show that the half life of cholesterol in the brain is 6 months, which means that the process of reducing brain cholesterol in vivo is a very slow process, and that any changes in Aβ resulting from decreased cholesterol are likely to be slow (19). Fassbender observed that treating guinea pigs with simvastatin for 3 weeks did not reduce cholesterol, but did reduce lathosterol (the precursor to cholesterol) and Aβ by about 50%. The reduction of Aβ occurring in absence of any change in cholesterol could be explained by a minor cholesterol compartment in neurons that changes more rapidly, but whose size is too small to be reflected in measures of total brain cholesterol. Alternatively, it is also possible that the critical species regulating Aβ production is another lipid in the cholesterol biosynthetic pathway. The study by Kojro et al. supports this possibility. They closely examined the relationship between cholesterol levels and α-secretase activity, and observed no significant increase in α-secretase until the reduction in cholesterol production is greater than 50%. Although α-secretase activity is a separate activity than Aβ production, this result raises the possibility that Aβ will decrease only when a threshold of cholesterol reduction is achieved. If this possibility is true, then why did simvastatin reduce Aβ production despite little, if any, change in total cholesterol levels? The answer might be that a precursor of cholesterol regulates Aβ production in vivo.
How cholesterol, or its precursors, affect protein function is one of the most interesting and controversial subjects in biochemistry today (20). The distribution of cholesterol throughout the membrane is not uniform. The cholesterol content of membranes increases as the membranes move from the endoplasmic reticulum through the Golgi apparatus to the plasma membrane. The cholesterol content of any given part of the membrane is also not uniform. Some patches of membrane, termed lipid rafts, contain high densities of cholesterol (Fig. 1C). Lipid rafts also contain high amounts of sphingolipids, which are a lipid that promotes the activity and stability of many membrane proteins. However, sphingolipids do not pack well. Cholesterol is used by the cell to enable orderly packing of the sphingolipids by intercalating between the sphingolipids. Hence, lipid rafts also have high cholesterol content. Because cholesterol is a rigid molecule, lipid rafts are regions of low membrane fluidity. By studying membrane fluidity, Kojro et al. observed evidence consistent with the complementary nature of Aβ and APPsα production. Sites of γ-secretase activity and Aβ production are associated with membrane regions of high cholesterol content, such as lipid rafts (Fig. 1C). In contrast, Kojro et al. show that sites of APPsα production occur in membrane regions with low cholesterol content and high fluidity (Fig. 1B). Thus, high membrane cholesterol content favors Aβ production and low membrane cholesterol content favors APPsα production.
Developing effective treatments for AD is clearly an important goal. As the specific mechanisms by which statins inhibit Aβ becomes understood, it might be possible to develop medicines that target the brain lipids or lipid compartments that specifically regulate Aβ production. This opens up new therapeutic approaches to AD. In the meantime, the discovery that cholesterol-lowering medicines, such as statins, can reduce Aβ production is particularly important because statins are already available clinically, and are safe medicines that have few side effects. The ability of statins to reduce Aβ production offers the exciting prospect that an existing medicine might be effective in delaying the onset or progression of AD.
Footnotes
References
- 1.Wolozin B, Kellman W, Ruosseau P, Celesia G G, Siegel G. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 2.Jick H, Zornberg G L, Jick S S, Seshadri S, Drachman D A. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 3.Refolo L M, Pappolla M A, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint G S, Sambamurti K, Duff K. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 4.Kojro E, Gimpl G, Lammich S, März W, Fahrenholz F. Proc Natl Acad Sci USA. 2001;98:5815–5820. doi: 10.1073/pnas.081612998. . (First Published April 17, 2001; 10.1073/pnas.081612998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fassbender K, Simons M, Bergmann C, Stroick M, Lütjohann D, Keller P, Runz H, Kühl S, Bertsch T, von Bergmann K, Hennerici M, et al. Proc Natl Acad Sci USA. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. . (First Published April 10, 2001; 10.1073/pnas.081620098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y M, Lai M T, Xu M, Huang Q, DiMuzio-Mower J, Sardana M K, Shi X P, Yin K C, Shafer J A, Gardell S J. Proc Natl Acad Sci USA. 2000;97:6138–6143. doi: 10.1073/pnas.110126897. . (First Published May 9, 2000; 10.1073/pnas.110126897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vassar R, Bennett B, Babu-Khan S, Kahn S, Mendiaz E, Denis P, Teplow D, Ross S, Amarante P, Loeloff R, et al. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 8.Koike H, Tomioka S, Sorimachi H, Saido T C, Maruyama K, Okuyama A, Fujisawa-Sehara A, Ohno S, Suzuki K, Ishiura S. Biochem J. 1999;343:371–375. [PMC free article] [PubMed] [Google Scholar]
- 9.Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Proc Natl Acad Sci USA. 1999;96:3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buxbaum J D, Liu K N, Luo Y, Slack J L, Stocking K L, Peschon J J, Johnson R S, Castner B J, Cerretti D P, Black R A. J Biol Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 11.Marcinkiewicz M, Seidah N G. J Neurochem. 2000;75:2133–2143. doi: 10.1046/j.1471-4159.2000.0752133.x. [DOI] [PubMed] [Google Scholar]
- 12.Wolozin B, Behl C. Arch Neurol. 2000;57:793–796. doi: 10.1001/archneur.57.6.793. [DOI] [PubMed] [Google Scholar]
- 13.Nohturfft A, Brown M S, Goldstein J L. J Biol Chem. 1998;273:17243–17250. doi: 10.1074/jbc.273.27.17243. [DOI] [PubMed] [Google Scholar]
- 14.Nye J S. Curr Biol. 1999;9:R118. doi: 10.1016/s0960-9822(99)80076-1. [DOI] [PubMed] [Google Scholar]
- 15.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm J, Schroeter E, Schrijvers V, Wolfe M, Ray W, et al. Nature (London) 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 16.Bodovitz S, Klein W L. J Biol Chem. 1996;271:4436–4440. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- 17.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti C, Simons K. Proc Natl Acad Sci USA. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Racchi M, Baetta R, Salvietti N, Ianna P, Franceschini G, Paoletti R, Fumagalli R, Govoni S, Trabucchi M, Soma M. Biochem J. 1997;322:893–898. doi: 10.1042/bj3220893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersson M, Elmberger P G, Edlund C, Kristensson K, Dallner G. FEBS Lett. 1990;269:15–18. doi: 10.1016/0014-5793(90)81107-y. [DOI] [PubMed] [Google Scholar]
- 20.Simons K, Ikonen E. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]