Abstract
Background
The purpose of this study was to characterize intraocular pressure (IOP) reduction throughout the day with travoprost ophthalmic solution 0.004% dosed once daily in the evening.
Methods
The results of seven published, randomized clinical trials including at least one arm in which travoprost 0.004% was dosed once daily in the evening were integrated. Means (and standard deviations) of mean baseline and on-treatment IOP, as well as mean IOP reduction and mean percent IOP reduction at 0800, 1000, and 1600 hours at weeks 2 and 12 were calculated.
Results
From a mean baseline IOP ranging from 25.0 to 27.2 mmHg, mean IOP on treatment ranged from 17.4 to 18.8 mmHg across all visits and time points. Mean IOP reductions from baseline ranged from 7.6 to 8.4 mmHg across visits and time points, representing a mean IOP reduction of 30%. Results of the safety analysis were consistent with the results from the individual studies for travoprost ophthalmic solution 0.004%, with ocular hyperemia being the most common side effect.
Conclusion
Travoprost 0.004% dosed once daily in the evening provides sustained IOP reduction throughout the 24-hour dosing interval in subjects with ocular hypertension or open-angle glaucoma. No reduction of IOP-lowering efficacy was observed at the 1600-hour time point which approached the end of the dosing interval.
Keywords: travoprost ophthalmic solution 0.004%, intraocular pressure reduction
Introduction
Intraocular pressure (IOP) is an important risk factor for the development and progression of glaucoma. In recent years, The Ocular Hypertension Treatment Trial has demonstrated that IOP reduction can prevent the development of glaucoma among individuals with ocular hypertension1,2 and can reduce the risk of glaucoma progression among subjects with both normal3–5 and elevated IOP.4 The impact of both short-term and long-term IOP variability on progression risk has also been explored, with many6–10 (but not all)11,12 studies finding a positive relationship between greater IOP variability and higher rates of glaucomatous progression or development.
The possibility that IOP variability may be a risk factor for glaucoma progression has stimulated a growing interest in the clinical evaluation and characterization of diurnal variability. A number of recent studies have challenged traditional thinking about IOP behavior. Historically, circadian IOP was believed to reach its peak values in the morning hours around the time of waking. These studies were typically conducted with subjects in the sitting position. When the physiologically more appropriate habitual position was studied, with subjects sitting during daytime hours and supine at night, a different IOP pattern emerged in which IOP peaked at night during sleeping hours in both healthy13–17 and glaucomatous individuals.13,17 Additionally, diurnal IOP patterns may not be conserved from day to day,18,19 and IOP fluctuations in fellow-eye pairs may be less well correlated than previously believed.16,20–22
Despite this growing body of research suggesting that IOP variability may predict future glaucoma progression, routine evaluation of circadian IOP in clinical practice remains a significant clinical challenge. Unlike for blood pressure or blood glucose levels, there are no inexpensive, easy-to-use devices for home or self-assessment of IOP. IOP measurement remains an in-office assessment, rendering characterization of individual IOP variability both expensive and time-consuming. Adding to the challenge of clinical IOP variability is the observation that most subjects experience their peak IOP after typical office hours.13,17
In light of the realization that consistent IOP reduction throughout the 24-hour period is important in preventing glaucomatous progression, coupled with the difficulties of assessing 24-hour IOP in routine clinical practice, there remains a need for therapy that provides consistent IOP reduction throughout the dosing interval with minimal peak and trough variability.
Travoprost ophthalmic solution 0.004% is a prostaglandin analog available throughout the global marketplace and indicated for IOP reduction in subjects with open-angle glaucoma or ocular hypertension. Travoprost ophthalmic solution has been formulated with a variety of preservatives. This paper reports the results of an integrated analysis of seven randomized clinical trials containing at least one travoprost 0.004% monotherapy arm,23–29 the goal of which is to characterize the consistency of IOP reduction throughout the travoprost dosing interval.
Materials and methods
This integrated analysis comprised data from seven peer-reviewed, published, prospective randomized trials. Each study was conducted at multiple clinical centers throughout the world, and each was reviewed and approved by the appropriate ethics committees. Because this integrated analysis utilized study-level data rather than subject-level data, no individual data were accessed or analyzed, and no further ethics committee approval was required.
The seven studies included in this integrated analysis were selected based on shared characteristics. All but one were registration trials and each was conducted according to the rigorous and robust methodology required by the US Food and Drug Administration and other international drug approval bodies; the sole nonregistration trial was a duration of action study conducted to registration trial standards. Phase IV studies were excluded because the design of these trials differs from those of the registration trials (ie, sample size, endpoints, and inclusion and exclusion criteria). Six of the included studies were registration trials for travoprost preserved with either benzalkonium chloride (Travatan®, Alcon Laboratories, Fort Worth, TX) or sofZia® (Travatan Z®, Alcon); one was a registration trial for a travoprost-timolol fixed combination (DuoTrav®, Alcon) that included a travoprost monotherapy arm in a contribution of elements design. All seven studies utilized travoprost in strict accordance with its labeling, employed identical dosing regimens, and all had previously been published. Because of their shared purpose and design, the subjects enrolled in these studies met similar eligibility criteria and are thus a substantially homogenous population taken as a whole. Table 1 lists the key design characteristics of the seven studies included in this integrated analysis.
Table 1.
Key design features of the seven studies included in this integrated analysis
| Reference | n, Travoprost 0.004% arm(s) ITT/PP | Study eye IOP criteria | Visits and time points |
|---|---|---|---|
| Netland et al29 | 197/187 | 24–36 mmHg at 0800 and 21–36 mmHg at 1000 and 1600 on two separate visits | 0800, 1000, and 1600 at baseline, week 2, and week 12 |
| Gross et al27 | 52/52 Travoprost 54/54 Travoprost with sofZia® |
24–36 mmHg at 0800 | 0800 at baseline and week 2 |
| Lewis et al28 | 341/339 Travoprost 338/322 Travoprost with sofZia® |
24–36 mmHg at 0800 and 21–36 mmHg at 1000 and 1600 on two separate visits | 0800, 1000, and 1600 at baseline, week 2, and week 12 |
| Goldberg et al26 | 197/176 | 24–36 mmHg at 0900 and 21–36 mmHg at 1100 and 1600 on two separate visits | 0900, 1100, and 1600 at baseline, week 2, and week 12 |
| Barnebey et al23 | 84/77 | ≥26 mmHg at 0800 on two separate visits, ≥24 mmHg at 1000 on one visit, and ≥22 mmHg at 1600 on one visit | 0800, 1000, and 1600 at baseline, week 2, and week 12 |
| Fellman et al24 | 197/179 | 24–36 mmHg at 0800 on 2 separate visits | 0800, 1000 and 1600 at baseline, week 2 and week 12 |
| Gandolfi et al25 | 185/177 Travoprost 185/176 Travoprost with sofZia® |
24–36 mmHg at 0900 and 21–36 at 1100 and 1600 on two separate visits | 0900, 1100 and 1600 at baseline, week 2 and week 12 |
Abbreviations: ITT, intent to treat; PP, per protocol; IOP, intraocular pressure.
The primary objective of this study was to characterize the consistency of IOP reduction achieved with travoprost 0.004% dosed once daily throughout the 24-hour dosing interval. The primary outcome of this descriptive analysis was the mean percent change from baseline in IOP at week 2 and week 12 at 0800, 1000 and 1600 hours (0900, 1100, and 1600 in European studies), which represent the latter half of the 24-hour dosing interval for travoprost. Not every study in the integrated analysis included IOP assessments at each of these visits and time points; as such, the sample size at each visit and time point was not constant.
For the mean and standard deviation, we exploited the fact that both of these parameters can be calculated for any collection of data using the sample size, the sum of the data values, and the sum of the squares of the data values. For a sample consisting of k values of the variable x, which we will denote as x1, x2, … xk, we have
and
where T denotes the sum of the x values, easily obtained by multiplying the sample mean by the sample size. For each study that contributed data to the integrated summary, working backwards from the sample size, mean, and standard deviation that had been provided, the formulae above were applied to calculate the sum of the data values and the sum of the squares of the data values for the study. These sums (and the sample sizes) were then added across all of the contributing studies in order to obtain the overall sample size, the sum of the combined data, and the sum of squares of the combined data. Applying the formulae above once again, the mean and standard deviation of the combined data were then obtained. Both the intent-to-treat and per protocol data sets were analyzed, and the results of both these analyses were similar. The per protocol data set is presented in this paper, because this integrated analysis is not a comparative study, and the per protocol data set is limited to those who were both randomized to and received travoprost 0.004% as study medication, and is therefore a more robust estimate of the efficacy of travoprost.
Results
Subject disposition and demographics
The integrated analysis consisted of data collected from a total of 1669 subjects, all of whom were included in the safety analysis. The intent-to-treat analysis included 1645 subjects who attended at least one on-treatment assessment. The per protocol analysis included 1563 subjects. Common reasons that subjects were excluded from the per protocol data set included failure to achieve required washout IOP level, use of excluded concomitant medications, nonadherence with study medication dosing, and other violations of eligibility criteria. Demographic and baseline characteristics of the integrated per protocol study population are given in Table 2.
Table 2.
Demographics and baseline characteristics of subjects in the per protocol data set (n = 1563) of the integrated analysis
| Age (years), mean (standard deviation) | 63.1 ± 11.3 |
| Gender, n (%) | |
| Male | 719 (46.0) |
| Female | 844 (54.0) |
| Ethnicity, n (%) | |
| Caucasian | 1130 (72.3) |
| Black | 247 (15.8) |
| Hispanic | 89 (5.7) |
| Asian | 41 (2.6) |
| Other | 56 (3.6) |
| Diagnosis, n (%) | |
| Open-angle glaucoma | 962 (61.5) |
| Ocular hypertension | 549 (35.1) |
| Pigmentary glaucoma | 27 (1.7) |
| Pseudoexfoliation glaucoma | 25 (1.6) |
Source data
The mean (± standard deviation) IOP at each visit and time point in the travoprost arms of each of the seven studies included in this integrated analysis are given in Table 3. These are the values from the per protocol analyses and therefore may differ from values in the published study reports which used intent-to-treat analyses in keeping with the design and statistical goals of those studies.
Table 3.
Mean (± standard deviation) intraocular pressure (mmHg) at each visit and time point in the travoprost arms of the seven studies included in this integrated analysis (per protocol data set)
| Reference | Baseline | Week 2 | Week 12 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| 0800 | 1000 | 1600 | 0800 | 1000 | 1600 | 0800 | 1000 | 1600 | |
| Netland et al29 | 26.8 (2.6) | 25.2 (2.8) | 24.6 (2.8) | 18.8 (3.6) | 17.52 (3.5) | 17.3 (3.0) | 18.7 (3.3) | 17.3 (3.7) | 17.6 (3.1) |
| Gross et al27 | |||||||||
| Travoprost | 27.1 (2.9) | – | – | 18.5 (4.0) | – | – | – | – | – |
| Travoprost with sofZia® | 26.9 (3.2) | – | – | 18.7 (4.2) | – | – | – | – | – |
| Lewis et al28 | |||||||||
| Travoprost | 27.2 (2.7) | 25.6 (2.9) | 24.9 (2.9) | 18.8 (3.5) | 17.9 (3.6) | 17.5 (3.5) | 18.8 (3.6) | 17.7 (3.3) | 17.2 (3.1) |
| Travoprost with sofZia® | 27.0 (2.3) | 25.5 (2.7) | 24.8 (2.7) | 18.5 (2.9) | 17.7 (3.3) | 17.3 (3.1) | 18.7 (3.5) | 17.7 (3.2) | 17.3 (3.2) |
| Goldberg et al26 | 27.4 (2.8) | 26.5 (2.9) | 25.6 (3.0) | 18.9 (3.4) | 17.9 (3.3) | 17.4 (3.3) | 18.5 (3.4) | 17.6 (3.1) | 16.8 (2.9) |
| Barnebey et al23 | 29.8 (2.7) | 28.2 (3.1) | 26.9 (3.5) | 20.6 (3.9) | 19.0 (3.8) | 18.6 (3.6) | 20.6 (3.7) | 19.2 (3.2) | 18.7 (3.2) |
| Fellman et al24 | 27.3 (3.0) | 25.7 (3.4) | 25.1 (3.0) | 19.3 (3.7) | 18.1 (3.4) | 17.6 (3.2) | 19.7 (3.9) | 18.5 (3.8) | 18.0 (3.3) |
| Gandolfi et al25 | 26.9 (2.6) | 25.6 (2.9) | 24.8 (2.8) | 18.1 (3.5) | 17.8 (3.5) | 17.1 (3.2) | 18.0 (3.5) | 17.4 (3.4) | 17.0 (3.7) |
Note: All studies evaluated travoprost (preserved with benzalkonium chloride) unless otherwise stated.
Consistency of IOP reduction
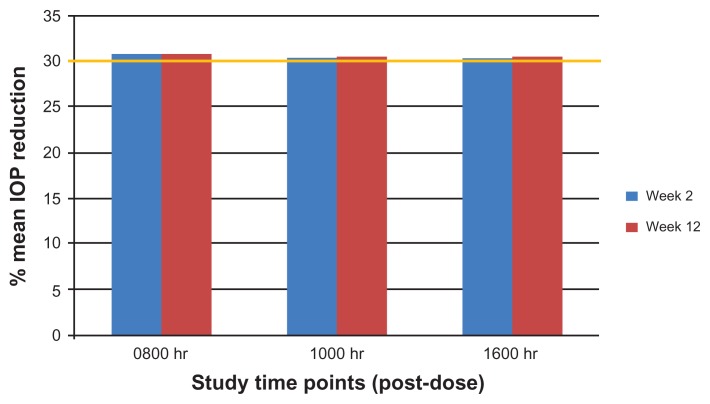
The baseline IOP and mean IOP, mean IOP reduction, and mean percent IOP reduction at each on-treatment visit and time point in the per protocol data set are given in Table 4. From mean baseline IOP ranging from 25.0 to 27.2 mmHg, mean IOP on treatment ranged from 17.4 to 18.8 mmHg across all visits and time points. Mean IOP reductions from baseline ranged from 7.6 to 8.4 mmHg across visits and time points, with less than a 1 mmHg difference between 0800 and 1600 time points at both weeks 2 and 12. Mean percent IOP reductions were remarkably consistent throughout the day. Mean IOP was lowered by at least 30% at every visit and time point, including the 1600-hour time point, 20 hours into the travoprost dosing interval (Figure 1).
Table 4.
Mean IOP at baseline and mean IOP, mean IOP reduction, and percent mean IOP reduction at each visit and time point in the per protocol data set of the integrated analysis
| Baseline | Week 2 | Week 12 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| 0800 | 1000 | 1600 | 0800 | 1000 | 1600 | 0800 | 1000 | 1600 | |
| IOP, mmHg | |||||||||
| Mean | 27.2 | 25.8 | 25.0 | 18.8 | 17.9 | 17.4 | 18.8 | 17.8 | 17.4 |
| SD | 2.7 | 3.0 | 2.9 | 3.5 | 3.5 | 3.3 | 3.6 | 3.4 | 3.2 |
| n | 1563 | 1456 | 1457 | 1539 | 1436 | 1435 | 1376 | 1375 | 1375 |
| IOP reduction, mmHg | |||||||||
| Mean | – | – | – | −8.4 | −7.9 | −7.6 | −8.5 | −7.9 | −7.6 |
| SD | – | – | – | 3.3 | 3.5 | 3.4 | 3.3 | 3.5 | 3.3 |
| n | – | – | – | 1539 | 1463 | 1435 | 1376 | 1375 | 1375 |
| Percent IOP reduction, % | |||||||||
| Mean | – | – | – | −30.8 | −30.4 | −30.0 | −30.9 | −30.5 | −30.1 |
| SD | – | – | – | 11.3 | 12.3 | 11.9 | 11.5 | 12.0 | 11.8 |
| n | – | – | – | 1539 | 1436 | 1435 | 1376 | 1375 | 1375 |
Abbreviations: IOP, intraocular pressure; SD, standard deviation.
Figure 1.
Mean percent intraocular pressure reduction by visit and time point.
Safety
Among the 1669 subjects in this integrated analysis, travoprost was generally well tolerated, and most adverse events were mild to moderate in severity and required no intervention. The discontinuation rate due to all adverse events was 3.2% (53 of 1666 subjects for whom discontinuation data were available). The adverse events seen in 1% or more of subjects are given in Table 5. Ocular hyperemia was the most common adverse event. In three of the studies, ocular hyperemia was identified as an adverse event if the study physician judged that it had increased by one or more points on a four-point (0–3) scale; in the remaining four studies, ocular hyperemia was identified as an adverse event if the subject reported it. The rate of physician-reported ocular hyperemia (increase of one unit on a four-point scale) was 38.8% (232/598 subjects), while the rate of subject-reported ocular hyperemia was 8.5% (91/1071 subjects). Other common adverse events included ocular itching, discomfort, pain, dry eye, foreign body sensation, and keratitis, each of which occurred at an incidence between 1%–5%.
Table 5.
Adverse events occurring at a rate of 1% or greater in the integrated analysis (n = 1669 subjects with safety data)
| n (%) | |
|---|---|
| Ocular hyperemia, physician-reported (n = 598/1669) | 232 (38.8) |
| Ocular hyperemia, subject-reported (n = 1071/1669) | 91 (8.5) |
| Ocular pruritus | 69 (4.1) |
| Ocular discomfort | 51 (3.1) |
| Ocular pain | 35 (2.1) |
| Dry eye | 33 (2.0) |
| Foreign body sensation | 33 (2.0) |
| Keratitis | 23 (1.4) |
Discussion
The current study demonstrates that travoprost ophthalmic solution 0.004% provides a sustained 30% IOP reduction. In this integrated analysis, travoprost exhibited no reduction of effect even in the latter portion of the 24-hour period. These results are consistent with the individual results of the included studies but provide a more robust estimate of the circadian IOP-lowering profile of travoprost in a sample of over 1500 subjects.
Consistent reduction of IOP throughout the 24-hour period is emerging as an important consideration in the management of glaucoma. An early study by Asrani et al brought attention to the role of IOP fluctuation in glaucoma progression.6 In that study, glaucoma subjects who had undergone diurnal IOP assessment several years previously, using a home tonometer initially designed for research applications, were assessed retrospectively for subsequent visual field progression. Subjects in the upper 25th percentile of diurnal IOP range were significantly more likely to experience glaucomatous progression compared with those in the lower 25th percentile, independent of mean IOP. More recently, a multicenter chart review found that the risk of progression increased by approximately 4–5-fold with each one unit increase in the standard deviation of intervisit IOP.7 While essentially no prospective studies have been designed specifically to evaluate the significance of diurnal variability on glaucoma progression, its importance can be inferred from analyses of many recent clinical trials. Post hoc analyses of cohorts of patients from both the Advanced Glaucoma Intervention Study8,9 and the Collaborative Initial Glaucoma Treatment Study10 data sets found associations between IOP variability and the risk of visual field progression. In contrast, the Early Manifest Glaucoma Study and Malmo Ocular Hypertension Study data sets did not confirm this association.11,12 One potential explanation for these disparate findings is that IOP variability may be less important in early-stage glaucoma compared with later-stage glaucoma.30 Given these reports, and despite the lack of appropriately designed and adequately powered studies directly exploring this topic, a preponderance of evidence to date suggests that IOP variability is relevant in the discussion of risk factors for glaucoma progression.
Our understanding of both diurnal IOP variability and long-term IOP fluctuation is limited by the paucity of studies on these topics, which in turn is attributable to the lack of home tonometry and the expense of collecting the many IOP measurements necessary to characterize IOP behavior over time. Peak IOP has recently been identified as an important IOP parameter associated with disease progression.31 IOP peaks at night when subjects are sleeping supine,13–17 making it impractical to determine peak IOP in routine clinical practice. Moreover, peak IOP may not occur at the same time on each day, because IOP patterns in both healthy and glaucomatous individuals are not highly conserved from day to day.18,19 Thus, assessing the efficacy of drug therapy in reducing an individual’s peak IOP is not currently feasible in clinical practice. In light of this important limitation, a drug that consistently lowers IOP at all time points throughout the dosing interval would have value in reducing both the diurnal IOP variability and the long-term fluctuation of IOP in subjects with open-angle glaucoma or ocular hypertension. The current analysis demonstrates that travoprost 0.004% dosed once daily in the evening provides a sustained mean IOP reduction of 30% throughout the day. Furthermore, this efficacy was consistent, being comparable at both week 2 and week 12 in the integrated analysis of seven clinical trials.
The clinical significance of a 30% IOP reduction is based on observations from major clinical trials. In the Ocular Hypertension Treatment Study, a 20% IOP reduction significantly reduced but did not eliminate the rate of conversion from ocular hypertension to open-angle glaucoma. 1 Similarly, an average 25% IOP reduction in the Early Manifest Glaucoma Trial significantly reduced but did not eliminate progression of manifest glaucoma.4 In contrast, subjects in both the medically and surgically treated arms of the Collaborative Initial Glaucoma Treatment Study demonstrated long-term stability of glaucoma, with mean IOP reductions of approximately 35% and 48%, respectively.32 These studies, taken together, suggest that the relationship between IOP reduction and glaucoma may take the form of a threshold effect; 25% reduction is not enough, and in excess of 35% may not be necessary for subjects with early or moderate open-angle glaucoma. We lack data on IOP reductions between 25%–35% to identify more precisely the optimal threshold IOP reduction.
The sustained 30% IOP reduction from baseline that can be achieved with travoprost monotherapy is a reasonable therapeutic target for most subjects with open-angle glaucoma or ocular hypertension. Some subjects, such as those with higher IOP, more advanced disease, or high-risk factors such as pseudoexfoliation, may require greater IOP reductions to achieve disease stability.
This analysis is strengthened by its large sample size, which permits robust estimation of both the efficacy and safety profiles of travoprost. It is also strengthened by inclusion of studies conducted to registration trial standards, thus ensuring that each study was adequately designed and powered and conducted under the strictest of protocols, each of which shared important methodological similarities such as eligibility criteria and IOP assessment. These key design features assure the integrity of both the efficacy and safety data collected in each study. The analysis is limited by the lack of nocturnal IOP data, which are not a standard component of registration trials for IOP-lowering medications. The data in this analysis cover only half the dosing interval, but it is the latter half, which represents a worst-case scenario when presumably efficacy approaches a trough effect.
This integrated analysis, with its large sample size for a prostaglandin analog study, allows a fuller characterization of the safety profile of travoprost. The safety profile identified in this analysis was consistent with the results of the individual studies, with hyperemia being the most common adverse event.
In summary, this integrated analysis demonstrates that travoprost 0.004% dosed once daily in the evening provides sustained 30% mean IOP reduction throughout the day in subjects with ocular hypertension or open-angle glaucoma. No significant loss of IOP-lowering efficacy was observed at the 1600-hour time point, which approached the end of the dosing interval.
Footnotes
Disclosure
Assistance with data analysis and preparation of this manuscript was provided by Douglas A Hubatsch (Global Medical Affairs, Alcon Laboratories Inc), Howard M Proskin (President, Howard M Proskin and Associates Inc), and Tony Realini.
References
- 1.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary openangle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 2.Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: the ocular hypertension treatment study. Arch Ophthalmol. 2010;128:276–287. doi: 10.1001/archophthalmol.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 4.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 5.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normaltension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 6.Asrani S, Zeimer R, Wilensky J, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9:134–142. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Lee PP, Walt JW, Rosenblatt LC, et al. Association between intraocular pressure variation and glaucoma progression: data from a United States chart review. Am J Ophthalmol. 2007;144:901–907. doi: 10.1016/j.ajo.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Caprioli J, Coleman AL. Intraocular pressure fluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology. 2008;115:1123–1129. e3. doi: 10.1016/j.ophtha.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 9.Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004;111:1627–1635. doi: 10.1016/j.ophtha.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Musch DC, Gillespie BW, Niziol LM, et al. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118:1766–1773. doi: 10.1016/j.ophtha.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bengtsson B, Leske MC, Hyman L, Heijl A. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:205–209. doi: 10.1016/j.ophtha.2006.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Bengtsson B, Heijl A. Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefes Arch Clin Exp Ophthalmol. 2005;243:513–518. doi: 10.1007/s00417-004-1103-8. [DOI] [PubMed] [Google Scholar]
- 13.Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44:1586–1590. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 14.Liu JH, Bouligny RP, Kripke DF, Weinreb RN. Nocturnal elevation of intraocular pressure is detectable in the sitting position. Invest Ophthalmol Vis Sci. 2003;44:4439–4442. doi: 10.1167/iovs.03-0349. [DOI] [PubMed] [Google Scholar]
- 15.Liu JH, Kripke DF, Twa MD, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40:2912–2917. [PubMed] [Google Scholar]
- 16.Liu JH, Sit AJ, Weinreb RN. Variation of 24-hour intraocular pressure in healthy individuals: right eye versus left eye. Ophthalmology. 2005;112:1670–1675. doi: 10.1016/j.ophtha.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Mosaed S, Liu JH, Weinreb RN. Correlation between office and peak nocturnal intraocular pressures in healthy subjects and glaucoma patients. Am J Ophthalmol. 2005;139:320–324. doi: 10.1016/j.ajo.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 18.Realini T, Weinreb N, Wisniewski S. Short-term repeatability of diurnal intraocular pressure patterns in glaucomatous individuals. Ophthalmology. 2011;118:47–51. doi: 10.1016/j.ophtha.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Realini T, Weinreb RN, Wisniewski SR. Diurnal intraocular pressure patterns are not repeatable in the short term in healthy individuals. Ophthalmology. 2010;117:1700–1704. doi: 10.1016/j.ophtha.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Realini T, Barber L, Burton D. Frequency of asymmetric intraocular pressure fluctuations among patients with and without glaucoma. Ophthalmology. 2002;109:1367–1371. doi: 10.1016/s0161-6420(02)01073-4. [DOI] [PubMed] [Google Scholar]
- 21.Sit AJ, Liu JH, Weinreb RN. Asymmetry of right versus left intraocular pressures over 24 hours in glaucoma patients. Ophthalmology. 2006;113:425–430. doi: 10.1016/j.ophtha.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Bhorade AM, Gordon MO, Wilson B, et al. Variability of intraocular pressure measurements in observation participants in the ocular hypertension treatment study. Ophthalmology. 2009;116:717–724. doi: 10.1016/j.ophtha.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnebey HS, Orengo-Nania S, Flowers BE, et al. The safety and efficacy of travoprost 0.004%/timolol 0.5% fixed combination ophthalmic solution. Am J Ophthalmol. 2005;140:1–7. doi: 10.1016/j.ajo.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 24.Fellman RL, Sullivan EK, Ratliff M, et al. Comparison of travoprost 0.0015% and 0.004% with timolol 0.5% in patients with elevated intraocular pressure: a 6-month, masked, multicenter trial. Ophthalmology. 2002;109:998–1008. doi: 10.1016/s0161-6420(02)01010-2. [DOI] [PubMed] [Google Scholar]
- 25.Gandolfi S, Paredes T, Goldberg I, et al. Comparison of a travoprost BAK-free formulation preserved with polyquaternium-1 with BAK-preserved travoprost in ocular hypertension or open-angle glaucoma. Eur J Ophthalmol. 2012;22:34–44. doi: 10.5301/ejo.5000001. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg I, Cunha-Vaz J, Jakobsen JE, et al. Comparison of topical travoprost eye drops given once daily and timolol 0.5% given twice daily in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2001;10:414–422. doi: 10.1097/00061198-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Gross RL, Peace JH, Smith SE, et al. Duration of IOP reduction with travoprost BAK-free solution. J Glaucoma. 2008;17:217–222. doi: 10.1097/IJG.0b013e31815a3472. [DOI] [PubMed] [Google Scholar]
- 28.Lewis RA, Katz GJ, Weiss MJ, et al. Travoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacy. J Glaucoma. 2007;16:98–103. doi: 10.1097/01.ijg.0000212274.50229.c6. [DOI] [PubMed] [Google Scholar]
- 29.Netland PA, Landry T, Sullivan EK, et al. Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2001;132:472–484. doi: 10.1016/s0002-9394(01)01177-1. [DOI] [PubMed] [Google Scholar]
- 30.Caprioli J, Varma R. Intraocular pressure: modulation as treatment for glaucoma. Am J Ophthalmol. 2011;152:340–344. e2. doi: 10.1016/j.ajo.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 31.De Moraes CG, Hill V, Tello C, et al. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma. 2011 Jun 21; doi: 10.1097/IJG.0b013e3182071b92. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 32.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]