Abstract
Purpose
To assess the relationship between postoperative endothelial cell loss and microcoaxial phaco parameters using Ozil IP (Alcon Laboratories, Inc, Fort Worth, TX) in noncomplicated cataract surgery.
Methods
In this prospective observational study, 120 consecutive cases of cataract patients with different grades of nuclear hardness underwent microcoaxial phacoemulsification through a 2.2-mm clear corneal incision. An Alcon Infinity Vision System with Ozil IP (Alcon Laboratories) was used with an Ozil torsional handpiece and a Kelman-style 45° phacoemulsification tip. Patients underwent preoperative and postoperative central endothelial cell counts.
Results
The study included 120 cases of age-related cataract whose mean age (standard deviation [SD]) was 59.68 years (9.47). There was a highly statistically significant endothelial cell loss (P < 0.001). The endothelial cell loss ranged 11–1149 cells/mm2 with a median (interquartile range) of 386 cells/mm2 (184.5–686 cells/mm2). The percentage of postoperative ECLoss% ranged from 0.48% to 47.8% with a median (interquartile range) of 15.4% (7.2% to 26.8%). A significant positive correlation was found between the ECLoss% and different phaco parameters. The Spearman’s rank-order correlation coefficient values, rho, (ρ) were as follows: CDE (ρ = 0.425), aspiration time (ρ = 0.176), and volume (ρ = 0.278). Also, ECLoss% was significantly correlated with the grade of nuclear opalescence (Kendall’s tau τ = 0.42).
Conclusion
Microcoaxial phacoemulsification was efficient in removing noncomplicated cataracts; however a statistically significant endothelial cell loss was noted, especially with increased nuclear hardness. This endothelial cell loss was mostly related to the increased cumulative dissipated energy (CDE), aspiration time, and volume of balanced salt solution used.
Keywords: cataract surgery, phacoemulsification, Ozil, endothelial cells, cumulative dissipated energy
Introduction
Cataract is one of the leading causes of preventable and curable blindness worldwide. Recently, especially with the advancement of technology, there has been a trend towards making cataract surgery not simply a procedure to remove the opaque lens, but additionally to aim at achieving the best possible visual outcome with optimal safety and minimum invasiveness. These goals have created a trend toward using a smaller wound during phacoemulsification that is associated with less surgically induced astigmatism, better fluidics, and phaco power modulation to allow for faster recovery with less tissue damage and inflammation. The problem facing ophthalmic surgeons after performing cataract surgery with intraocular lens implantation may be corneal decompensation due to endothelial cell loss.1
Torsional ultrasound (US) using a torsional handpiece that produces side-to-side rotary oscillations has been recently introduced.2,3 Torsional US has a lower resonant frequency and slower needle movement than conventional US, which allows for optimized cutting efficiency with reduced heat generation.2,4,5 Torsional US at different vacuum levels, like conventional US, leads to differences in parameters such as phaco time, US efficiency, and endothelial cell changes.
The corneal endothelium is a single layer of polygonal cells lining the back surface of the cornea. Relative corneal dehydration and transparency is controlled by the active endothelial ionic pumps, which maintain a low level of stromal hydration.6 In the human eye, the endothelial cell density decreases with aging from 4000 cells/mm2 in childhood to approximately 2500 cells/mm2 at age 80 years.7 When the endothelial cell count drops below 600 to 800 cells/mm2, corneal decompensation, and corneal edema occur as a result of the compromised pump function. Endothelial cells are non-replicative, and cell loss is compensated by enlargement and migration of residual cells.6,8,9 This naturally occurring process is exacerbated when there is additional cell loss resulting from intraocular surgery, caused by both the heat generated and the turbulence of fluids within the anterior chamber that occur during phaco surgery. Despite the fact that cases with low endothelial cell density (ECD) could be at greater risk of developing corneal decompensation, no significant correlation was found between the preoperative ECD and the percentage of postoperative endothelial cell loss.10
Other factors might also influence postoperative endothelial cell loss, such as the nature of the irrigating solution and its volume. There is a trend towards lower postoperative endothelial cell density for surgeries with longer phacoemulsification time and higher irrigation volumes if Ringer’s solution is used instead of balanced salt solution (BSS).11
The current study was carried out to assess the relationship between postoperative endothelial cell loss and different microcoaxial phaco parameters using Ozil Intelligent Phaco (IP) in noncomplicated cataract surgery.
Patients and methods
Patients
One hundred and twenty patients with cataract were consecutively recruited. Table 1 shows the patients’ demographic characteristics. The participants underwent phacoemulsification using the Infiniti Vision System (Alcon, Laboratories Inc, Fort Worth, TX) with the Ozil IP platform.
Table 1.
Summary of the patients’ demographic data
| Age: mean (SD) | 59.68 (9.47) |
| Sex: number (percentage) | |
| Male | 57 (47.5%) |
| Female | 63 (52.5%) |
| Nuclear opalescence: number (percentage) | |
| Grade-I | 13 (10.83%) |
| Grade-II | 23 (19.17%) |
| Grade-III | 39 (32.50%) |
| Grade-IV | 24 (20.00%) |
| Grade-V | 21 (17.50%) |
| Preoperative VA: mean (SD) LogMar | 1.42 (0.54) |
| Postoperative VA: mean (SD) LogMar | 0.32 (0.19) |
Abbreviations: VA, visual acuity; SD, standard deviation.
Patients with nuclear or corticonuclear cataract of grades I–V according to the Lens Opacities Classification System III (LOCS III) scale were included in the study.12 Exclusion criteria included: cataract hardness greater than nuclear opalescence (NO) 5 on the LOCS III scale; coexisting ocular disease; pseudoexfoliation; and poor pupillary dilatation. Corneal exclusion criteria included corneal dystrophy, corneal scarring, and an endothelial cell count (ECC) less than 1500 cells/mm2. Cases with age-related macular degeneration and glaucoma were excluded and eyes with a history of trauma or surgery were excluded as well.
The research followed the tenets of the Declaration of Helsinki after approval of the study protocol by The Scientific and research committee of Directorate of Health Services, South Batinah Region. Informed consent was obtained from all patients enrolled in the study after the nature and possible consequences of the procedures had been explained.
Preoperative examinations
A complete ocular examination, biometry for intraocular lens (IOL) power calculation, and an endothelial cell count (ECC) were performed preoperatively (ECC-pre) and postoperatively at 3 months (ECC-post). Keratometric power was obtained using a Bausch and Lomb manual keratometer (Bausch and Lomb, Rochester, NY) and axial length was measured using an ultrasonic A-Scan (Alcon Laboratories).
Central corneal ECC was performed using a noncontact Tomey specular microscope EM 3000 (Tomey Corp, Nagoya, Japan).
The percentage of postoperative endothelial cell loss (ECLoss%) was calculated as follows:
where ECLoss% is the percentage of postoperative endothelial cell loss, ECC-pre is the preoperative cell count, and ECC-post is the postoperative corneal endothelial cell count 3 months postoperatively.
Anesthesia
Local peribulbar anesthesia was used in all patients, where a 50/50 mix of 2% lidocaine (Pharmaceutical Solution Industries, Jeddah, Saudi Arabia) and 0.5% bupivacaine (Hospira Inc, Lake Forest, IL) was used. Cyclopentolate was applied topically 60, 45, 30, and 15 minutes before surgery to dilate the eye. The surgery then proceeded as scheduled and all patients received subconjunctival injection of dexamethasone plus gentamycin at the end of the surgery.
Surgical technique
Microcoaxial phacoemulsification was performed with an Ozil Torsional Handpiece and 0.9-mm mini-flare Aspiration Bypass System (ABS) 45° Kelman Tip using the Infiniti Vision System (all from Alcon Laboratories, Inc, Fort Worth, TX) with the Ozil IP platform. Micro- Smooth ULTRA Infusion sleeves were used in all cases. Infusion bottle height was set between 90–110 cm, guided by the case situation and surgeons’ preferred technique especially during fragment removal. The aspiration flow rate was 27–40 mL/min and the vacuum level was set to 350–450 mmHg. All cases were operated by either MAM or AH. The Alcon INTREPID Fluidic Management System (FMS) with noncompliant tubing was used to provide enhanced chamber stability. A standard clear corneal (at the steep meridian) incision was performed using a 2.2-mm ClearCut blade (Alcon Laboratories). One or two side ports 180° apart were created with a 1.0-mm ClearCut blade. A dispersive ophthalmic viscosurgical device (OVD), sodium hyaluronate 3.0%:chondroitin sulfate 4.0% (Viscoat®, Alcon Laboratories (SA) Pty Ltd, Randberg, South Africa) was injected into the anterior chamber followed by injection of high-molecular-weight sodium hyaluronate 1.0% (Provisc®, Alcon Laboratories (SA), Pty Ltd) during the earlier phase of surgery. Later on, during subsequent phases of surgery a cohesive OVD, Provisc® was used to deepen the anterior chamber and to expand the capsular bag for implantation of the intraocular lens. Continuous curvilinear capsulorhexis was made using a bent 25-gauge insulin needle. Hydrodissection was done using the same viscoelastic canula in all cases. The nucleus was then rotated. A standard phacoemulsification technique was used in which the Ozil torsional handpiece and Kelman-style curved 45° phacoemulsification tip is used to sculpt a standard central vertical groove 80%–90% deep along the full extent of the nucleus. The nucleus was then cracked into two halves and each half was then chopped using a stop and chop technique followed by emulsification and removal while in the posterior plane within the capsular margins. This was followed by cortical clean-up using either coaxial or bimanual I/A handpieces and the capsular bag was expanded with cohesive OVD followed by Acrysof posterior chamber intraocular lens insertion using a Monarch III injector and D-cartridge system (Alcon Laboratories). This was followed by subsequent removal of OVD and hydration of the wound and side ports.
The routine postoperative treatment was as follows: topical prednisolone acetate 1% eyedrops 2-hourly for the first 3 days then 3-hourly for another 3 days, then 4-hourly for 10 days. Topical ofloxacin 0.3% drops were used 4 times/day. One capsule of 100 mg sustained release nonsteroidal anti-inflammatory drug (diclofenac sodium) was used for 5 days in all cases.
During surgery the different phaco parameters values were automatically calculated by the Infinity Vision System and displayed on the monitor. At the end of surgery the Metrics dialog box was activated and the metrics readouts were then collected and subjected for analysis.
The different phaco parameters used were defined in the machine manual by Alcon:
U/S Total Time: sum of phaco time and torsional time.
U/S Total Equivalent Power in Position 3: = CDE/(U/S Total Time)
Cumulative Dissipated Energy: Total U/S energy in footpedal position 3 (both phaco and torsional) and was calculated as: (phaco time × average phaco power) + (torsional time × 0.4 × average torsional amplitude). The factor 0.4 represents an approximate reduction of heat dissipated at the incision as compared to conventional phaco.
Aspiration Time: Total time the system was aspirating in footpedal position 2 or 3 for U/S and I/A.
Estimated Fluid used (volume): an estimation of the volume of fluid aspirated during the procedure based on system setting and time.
Statistical analysis
SPSS software (v 16.0; SPSS Inc, Chicago, IL) was used to perform statistical analysis. A significance was taken at P value ≤ 0.05. The following variables were correlated after being tested for normal and abnormal distribution, endothelial cell loss and LOCS III NO grade, and different phaco parameters including: phacoemulsification time; torsional total time; average phaco power; average phaco power in position 3; cumulative dissipated energy (CDE); aspiration time; and estimated volume of BSS used during the procedure (volume). Multiple linear regression analyses were performed to determine whether age, nuclear opacity, phacoemulsification time, or CDE predicted corneal endothelial cell loss.
The Pearson’s product-moment correlation coefficient (r) was used to evaluate the relationship between approximately normally distributed continuous variables. Spearman’s rank-order correlation coefficient, rho, (ρ) was used to evaluate the relationship between continuous variables where at least one of them is not normally distributed. Kendall’s rank-correlation coefficient, tau (τ), was used to evaluate the relationship between ordinal (nuclear opalescence grade) and continuous variables.
Results
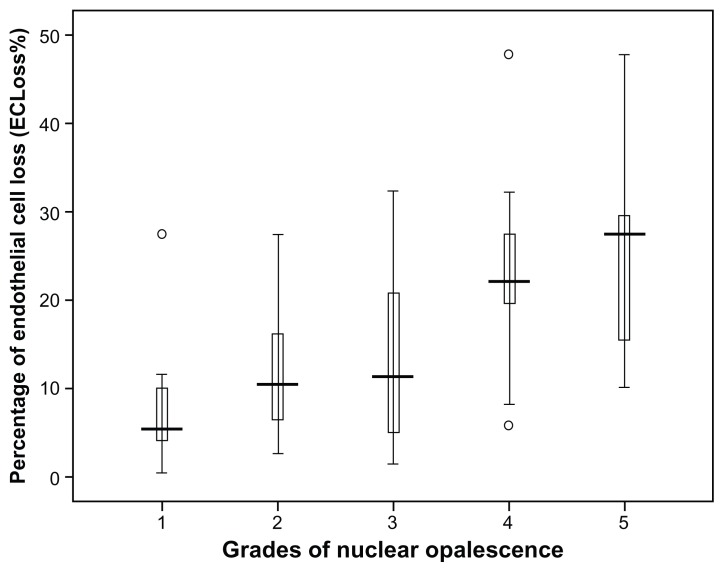
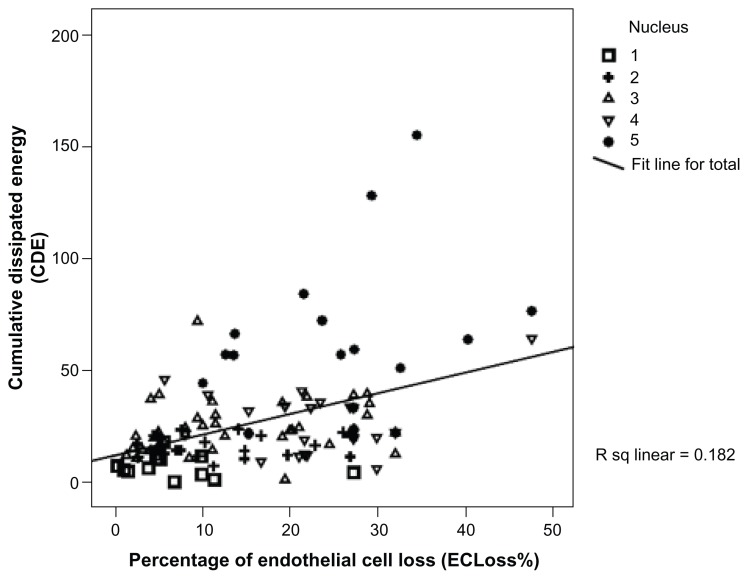
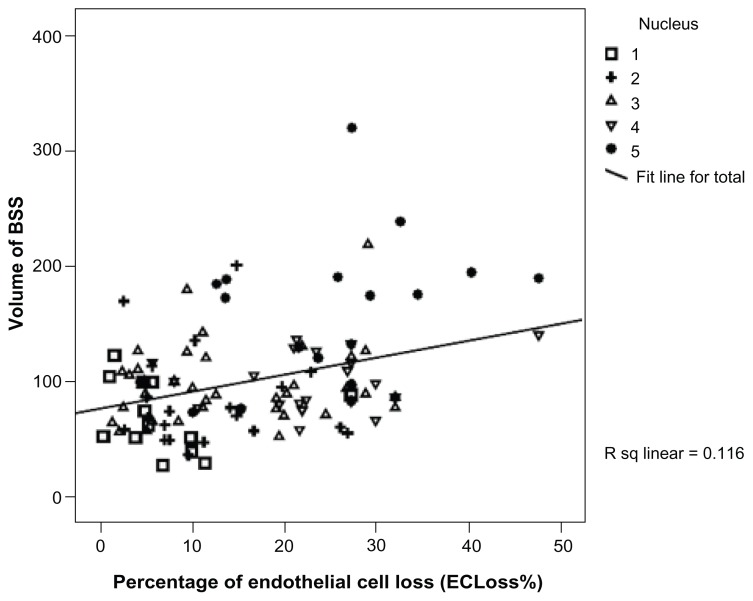
The current study included 120 cases of age-related cataract whose mean age (SD) was 59.68 years (9.47). Males constituted 47.5% (57/120) of the group and females 52.5% (63/120). Table 1 summarizes the patients’ demographic characteristics while Table 2 shows the summary of the different phaco parameters. Postoperative findings revealed a highly statistically significant endothelial cell loss (P < 0.001). The endothelial cell loss varied from 11 to 1149 cells/mm2 with a median (interquartile range) of 386 cells/mm2 (184.5–686 cells/mm2). Postoperative ECLoss% ranged from 0.48% to 47.8% with a median (interquartile range) of 15.4% (7.2% to 26.8%) according to the grade of nuclear opalescence as shown in Figure 1. Table 3 summarizes the correlation between the percentage of postoperative ECLoss% and various phaco parameters. A strong positive significant correlation was found between ECLoss% and many of the phaco parameters as follows: the Spearman’s rank-order correlation coefficient, rho, (ρ) values were CDE (ρ = 0.425), torsional time (ρ = 0.334), average phaco power (ρ = 0.307), average phaco power in position 3 (ρ = 0.343), aspiration time (ρ = 0.176), volume (ρ = 0.278), and phaco time (ρ = 0.345). Figure 2 illustrates the correlation between ECLoss% and CDE, while Figure 3 shows the correlation with the volume of BSS used. Also, ECLoss% was not significantly correlated with age. Table 4 shows the correlation between the different grades of nuclear opalescence (according to LOCS III) and different phaco parameters. Kendall’s rank-correlation coefficient, tau (τ), was highly significant for the relationship between ECLoss% and the grade of nuclear opalescence [τ] = 0.42. Figure 1 illustrates the variation in percentage ECLoss% with different grades of nuclear opalescence.
Table 2.
Summary of patient age, pre and postoperative visual acuity (LOGMAR) and the different phaco parameters
| N | Mean | Std. Dev | Mini | Max | Percentiles | |||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 25th | 50th (median) | 75th | ||||||
| Pre-Op BCVA | 120 | 1.42 | 0.55 | 0.1 | 2 | 1 | 1.3 | 2 |
| Post-Op BCVA | 120 | 0.33 | 0.19 | 0 | 0.8 | 0.18 | 0.3 | 0.48 |
| Age | 120 | 59.68 | 9.47 | 40 | 88 | 53 | 60 | 65 |
| US Tot. Eq. P/3 | 120 | 24.80 | 68.43 | 2.60 | 65.00 | 13.73 | 17.50 | 23.53 |
| Phaco time | 120 | 11.76 | 22.79 | 0 | 149 | 0 | 3 | 11 |
| Average. Phaco Power | 120 | 15.53 | 14.57 | 0 | 63.5 | 1.975 | 11.65 | 26.35 |
| Average_Phaco_Power/3 | 119 | 1.52 | 3.23 | 0 | 18.8 | 0.1 | 0.3 | 1.5 |
| Torsional time | 120 | 74.42 | 58.00 | 0 | 363 | 38.25 | 61 | 95.75 |
| Eq. Average. Tor. Ampl/3 | 120 | 17.50 | 7.87 | 0 | 55.7 | 12.4 | 16.5 | 22.55 |
| Aspiration time | 120 | 6.20 | 2.90 | 1.56 | 16.27 | 4.0325 | 5.35 | 7.987 |
| Volume | 120 | 99.41 | 45.62 | 26 | 318 | 73 | 88 | 119 |
| CDE | 120 | 27.52 | 23.01 | 0.47 | 155.4 | 13.23 | 21.725 | 34.202 |
| ECC-pre | 120 | 2527.12 | 186.43 | 1969 | 2925 | 2429 | 2501 | 2680.5 |
| ECC-post | 120 | 2101.39 | 281.58 | 1254 | 2722 | 1941.25 | 2088 | 2319 |
| ECLoss | 120 | 425.73 | 274.48 | 11 | 1149 | 184.5 | 386 | 686 |
| ECLoss% | 120 | 16.68 | 10.62 | 0.48 | 47.82 | 7.18 | 15.44 | 26.782 |
Abbreviations: ECC-pre, preoperative endothelial cell count; ECC-post, postoperative endothelial cell count; ECLoss, endothelial cell loss; ECLoss%, percentage of endothelial cell loss; US Tot. Eq. P/3, ultrasonic total equivalent power in position 3; average_Phaco_Power/3, average phaco power in position 3; Eq. Avg. Tor. Ampl/3, equivalent average torsional amplitude in position 3; CDE, cumulative dissipated energy; pre-op BCVA, preoperative best corrected visual acuity (LOGMAR); Post-Op BCVA, postoperative best corrected visual acuity (LOGMAR).
Figure 1.
The chart shows the variation in the percentage of endothelial cell loss (ECLoss%) with varying grades of nuclear opalescence according to the LOCS III system.
Abbreviations: ECLoss%, percentage of endothelial cell loss; LOCS III, Lens Opacities Classification System III.
Table 3.
Correlation matrix showing the correlation between ECLoss% (percentage of postoperative endothelial cell loss) at 3 months and different phaco parameters
| Age | CDE | Torsional T. | Volume | Phaco T. | Average Phaco Power | Average Phaco Power/3 | Aspiration T. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Spearman’s rho | ECLoss% | Correlation coefficient | −0.077 | 0.425 | 0.334 | 0.278 | 0.345 | 0.307 | 0.343 | 0.176 |
| Sig. (2-tailed) | 0.406 | 0.000 | 0.000 | 0.002 | 0.000 | 0.001 | 0.000 | 0.054 | ||
| N | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 |
Note: Spearman’s rank-order correlation coefficient, rho, (ρ) was used to evaluate the relationship because at least one of the continuous variables is not normally distributed.
Abbreviations: ECLoss%, percentage of endothelial cell loss; US Tot. Eq. P/3, ultrasonic total equivalent power in position 3; Avg_Phaco_Power/3, Average phaco power in position 3; Eq. Avg. Tor. Ampl/3, Equivalent average torsional amplitude in position 3; CDE, cumulative dissipated energy. Torsional T, torsional time, Aspiratio T, Aspiration time; Phaco T, phaco time.
Figure 2.
Chart showing the correlation between the percentage of endothelial cell loss (ECLoss%) and cumulative dissipated energy (CDE).
Abbreviations: ECLoss%, percentage of endothelial cell loss; CDE, cumulative dissipated energy.
Figure 3.
Chart showing the correlation between the percentage of endothelial cell loss (ECLoss%) and volume of BSS used during surgery.
Abbreviations: ECLoss%, percentage of endothelial cell loss; BSS, balanced salt solution.
Table 4.
Correlation matrix showing the relationship between nuclear opalescence and different parameters
| CDE | Torsional T. | Average Phaco Power | Average Phaco Power/3 | Volume | Aspiration T. | Phaco T. | ECLoss% | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Kendall’s tau_b | Nuclear opalescence | Correlation coefficient | 0.52 | 0.41 | 0.20 | 0.26 | 0.45 | 0.28 | 0.36 | 0.42 |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.003 | 0.000 | 0.00 | 0.000 | 0.000 | 0.000 | ||
| Number | 120 | 120 | 120 | 120 | 120 | 120 | 120 | 120 |
Notes: Kendall’s rank-correlation coefficient, tau, (τ) was used to evaluate the relationship between ordinal (nuclear opalescence grade) and continuous variables.
Abbreviations: ECLoss%, percentage of endothelial cell loss; US Tot. Eq. P/3, ultrasonic total equivalent power in position 3; Avg_Phaco_Power/3, Average phaco power in position 3; Eq. Avg. Tor. Ampl/3, equivalent average torsional amplitude in position 3; CDE, cumulative dissipated energy. Torsional T, torsional time, Aspiratio T, Aspiration time; Phaco T, phaco time.
Discussion
The results of this study showed that it is possible to efficiently and safely remove cataract using torsional micro-coaxial phacoemulsification. However, concerns about the possibility of endothelial loss were raised. The study revealed that there is a relatively wide range of endothelial cell loss (ECLoss) that increases with increasing grade of nuclear hardness. It also revealed that a high CDE (Figure 2) and large volume of BSS used during phaco (Figure 3) was associated with a high ECLoss%.
Conflicting reports had found different ECLoss% values that varied between 7% for conventional and 11.6% for bimanual phaco.8,13 Other reports cited a 3-month postoperative endothelial cell loss between 4.6% and 15.6%.14–17 A reduction of 20%–30% in endothelial cell loss after phaco was also reported by Storr-Paulsen et al.18 The source of conflict in these studies might be attributed to variation in the patients’ selection criteria and the technique and phaco technology used.
Our study results that revealed a significant ECLoss% which was correlated with the nuclear hardness are in agreement with earlier reports by Vasavada et al and Lee et al.19,20
In a study by Lee et al,20 the percentage of endothelial cell loss was strongly correlated with the grade of nuclear opacity with a mean of 9.97% (for grade II) to 12.03% (for grade IV). The difference in the ECLoss% in our study could be attributed to the fact that our study included a higher grade of nuclear hardness (17.5% of our cases were grade V) which was not the case in the study by Lee et al (they included only cases of grade IV or less), while Vasavada et al19 had used a different system for grading nuclear hardness. Also, in the study by Lee et al,20 the CDE and the volume of BSS were positively correlated with the grade of nuclear opacity.
The study also revealed a strong positive correlation between ECLoss% and different phaco parameters as shown in Table 3 and Figures 2 and 3. The CDE is the parameter in which the total US energy in footpedal position 3 was considered (both phaco and torsional) as calculated earlier in the methods section. A higher CDE had been found to be associated with a significant loss of corneal endothelial cells. Not only that, but also, the volume of BSS used during phaco was associated with a higher ECLoss%, which was similar to the findings of Lucena et al.11 Despite the reduced energy generated while using torsional phaco, a high CDE associated with significant loss of endothelial cells was found, which means that in cases of denser cataracts the amount of energy reduction at the phaco tip is not enough to save the endothelium. Also, with increasing density of cataract there was a need for more time and increased amounts of BSS solution to remove the lens, with associated turbulence of fluid within the anterior chamber and consequently more endothelial cell loss. These findings were close to that found by Berdahl et al.21
The current study also showed a highly significant correlation between ECLoss% and aspiration time (ρ = 0.176), and BSS volume (ρ = 0.278). This could indicate that even in cases where there is not much energy used but instead the aspiration time is prolonged with consequent consumption of more BSS, the associated anterior chamber turbulence of fluids could also participate in damaging the corneal endothelium.
The limitation of this study is that it included a heterogenous group of cases with variable grades of nuclear hardness; a high percentage of them were grades IV–V on the LOCS III scale (37% of cases). This might account for the high percentage of postoperative endothelial cell loss.
Considering the above limitation we could conclude that microcoaxial phacoemulsification is efficient in removing noncomplicated cataracts; however a significantly higher postoperative endothelial cell loss was noted, especially with increased nuclear hardness. This endothelial cell loss was mostly related to the increased CDE, prolonged aspiration time, and increased amount of fluid used during the surgical procedure. Even with the use of torsional phaco, cataracts with increasing nuclear hardness require more phaco power (as torsional power alone is not highly efficient in hard cases) and consequently increased CDE that might end with higher endothelial cell loss. Prolonged aspiration time and the use of large amounts of BSS during phaco for any reason might also end with endothelial cell damage (even in cases with less nuclear hardness). All these factors should be carefully considered during phaco, especially in cases with compromised corneal endothelium, as corneal decompensation might occur.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kohnen T. Compromised corneal endothelium and cataract: how should we decide? J Cataract Refract Surg. 2011;37(8):1377–1378. doi: 10.1016/j.jcrs.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Zeng M, Liu X, et al. Torsional mode versus conventional ultrasound mode phacoemulsification: randomized comparative clinical study. J Cataract Refract Surg. 2007;33(2):287–292. doi: 10.1016/j.jcrs.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 3.Zeng M, Liu X, Liu Y, et al. Torsional ultrasound modality for hard nucleus phacoemulsification cataract extraction. Br J Ophthalmol. 2008;92(8):1092–1096. doi: 10.1136/bjo.2007.128504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davison JA. Cumulative tip travel and implied followability of longitudinal and torsional phacoemulsification. J Cataract Refract Surg. 2008;34(6):986–990. doi: 10.1016/j.jcrs.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Miyoshi T, Yoshida H. Ultra-high-speed digital video images of vibrations of an ultrasonic tip and phacoemulsification. J Cataract Refract Surg. 2008;34(6):1024–1028. doi: 10.1016/j.jcrs.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Wilczynski M, Drobniewski I, Synder A, Omulecki W. Evaluation of early corneal endothelial cell loss in bimanual microincision cataract surgery (MICS) in comparison with standard phacoemulsification. Eur J Ophthalmol. 2006;16(6):798–803. doi: 10.1177/112067210601600603. [DOI] [PubMed] [Google Scholar]
- 7.Werblin TP. Long-term endothelial cell loss following phacoemulsification: model for evaluating endothelial damage after intraocular surgery. Refract Corneal Surg. 1993;9(1):29–35. [PubMed] [Google Scholar]
- 8.Mencucci R, Ponchietti C, Virgili G, Giansanti F, Menchini U. Corneal endothelial damage after cataract surgery: microincision versus standard technique. J Cataract Refract Surg. 2006;32(8):1351–1354. doi: 10.1016/j.jcrs.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 9.Storr-Paulsen A, Norregaard JC, Farik G, Tarnhoj J. The influence of viscoelastic substances on the corneal endothelial cell population during cataract surgery: a prospective study of cohesive and dispersive viscoelastics. Acta Ophthalmol Scand. 2007;85(2):183–187. doi: 10.1111/j.1600-0420.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi K, Yoshida M, Manabe S, Hirata A. Cataract surgery in eyes with low corneal endothelial cell density. J Cataract Refract Surg. 2011;37(8):1419–1425. doi: 10.1016/j.jcrs.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Lucena DR, Ribeiro MS, Messias A, Bicas HE, Scott IU, Jorge R. Comparison of corneal changes after phacoemulsification using BSS Plus versus Lactated Ringer’s irrigating solution: a prospective randomised trial. Br J Ophthalmol. 2011;95(4):485–489. doi: 10.1136/bjo.2009.172502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chylack LT, Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111(6):831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 13.Prakash P, Kasaby HE, Aggarwal RK, Humfrey S. Microincision bimanual phacoemulsification and Thinoptx implantation through a 1.70 mm incision. Eye (Lond) 2007;21(2):177–182. doi: 10.1038/sj.eye.6702153. [DOI] [PubMed] [Google Scholar]
- 14.Tsuneoka H, Shiba T, Takahashi Y. Ultrasonic phacoemulsification using a 1.4 mm incision: clinical results. J Cataract Refract Surg. 2002;28(1):81–86. doi: 10.1016/s0886-3350(01)01235-4. [DOI] [PubMed] [Google Scholar]
- 15.Donnenfeld ED, Olson RJ, Solomon R, et al. Efficacy and wound-temperature gradient of whitestar phacoemulsification through a 1.2 mm incision. J Cataract Refract Surg. 2003;29(6):1097–1100. doi: 10.1016/s0886-3350(02)01917-x. [DOI] [PubMed] [Google Scholar]
- 16.Alio J, Rodriguez-Prats JL, Galal A, Ramzy M. Outcomes of microincision cataract surgery versus coaxial phacoemulsification. Ophthalmology. 2005;112(11):1997–2003. doi: 10.1016/j.ophtha.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 17.Reuschel A, Bogatsch H, Barth T, Wiedemann R. Comparison of endothelial changes and power settings between torsional and longitudinal phacoemulsification. J Cataract Refract Surg. 2010;36(11):1855–1861. doi: 10.1016/j.jcrs.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 18.Storr-Paulsen A, Norregaard JC, Ahmed S, Storr-Paulsen T, Pedersen TH. Endothelial cell damage after cataract surgery: divide-and-conquer versus phaco-chop technique. J Cataract Refract Surg. 2008;34(6):996–1000. doi: 10.1016/j.jcrs.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Vasavada V, Raj SM, Vasavada AR. Intraoperative performance and postoperative outcomes of microcoaxial phacoemulsification. Observational study. J Cataract Refract Surg. 2007;33(6):1019–1024. doi: 10.1016/j.jcrs.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Lee KM, Kwon HG, Joo CK. Microcoaxial cataract surgery outcomes: comparison of 1.8 mm system and 2.2 mm system. J Cataract Refract Surg. 2009;35(5):874–880. doi: 10.1016/j.jcrs.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Berdahl JP, Jun B, DeStafeno JJ, Kim T. Comparison of a torsional handpiece through microincision versus standard clear corneal cataract wounds. J Cataract Refract Surg. 2008;34(12):2091–2095. doi: 10.1016/j.jcrs.2008.08.025. [DOI] [PubMed] [Google Scholar]